These factors induce the sensation of dyspnoea via a complex interplay between chemoreceptor stimulation, mechanical abnormalities in breathing and the perception of those abnormalities by the central nervous system.3 In some cases, panic and fear relating to difficulty breathing, or “air hunger”, may further enhance the patient’s physical distress.3
In patients receiving palliative care, the initial aims of management are to treat any underlying conditions that may be aggravating their ability to breathe, and therefore reduce the level of dyspnoea.1 However, as the patient enters their last days of life, the goal of treatment transitions to alleviating the perception of dyspnoea via symptom relief. Treatment should begin with non-pharmacological interventions, then consider the addition of low-dose morphine, and if appropriate, benzodiazepines (if anxiety is a factor in their symptoms).2, 4 An individualised approach is required as treatment effectiveness varies between patients.
Assessing the patient’s condition
The patient’s own evaluation of their breathing is the most effective tool for assessing dyspnoea.2 Patients may describe the feeling as not getting enough oxygen with each breath, having to work harder to breathe or chest tightness.3 Clinical signs indicative of dyspnoea include tachypnoea, pursed lip breathing, gasping or wheezing, increased intercostal accessory muscle use and tachycardia.2 Chest auscultation may reveal abnormal sounds, e.g. rhonchi or crackles.5
As the end of life approaches, the ability to communicate effectively is often lost. In patients who have limited or no communication, look for signs of distress in addition to the symptoms and signs mentioned above, e.g. sweating, agitation and facial expression.6 Ask family/whānau about any noticeable changes in the patient’s condition.
Non-pharmacological interventions should initially be prioritised for managing dyspnoea in patients in their last days of life.7 The acceptability and effectiveness of interventions will depend on individual patient circumstances and preference. Ideally, many of these strategies will have been recommended and discussed with the patient and their family/whānau prior to the last days of life so that a clear and agreed upon plan is already in place (i.e. advance care planning, see: www.hqsc.govt.nz/our-work/advance-care-planning/).
Non-pharmacological strategies aimed at improving patient comfort include:
- Positioning the patient so that their head and torso are elevated. 2 An upright position is preferred* but patients in their last days of life may be unable to maintain this position. Use pillows to support the patient’s head, neck, torso and arms.6 Horseshoe pillows (“tri-pillows”) should be avoided due to a risk of increased respiratory difficulty if a smaller patient is incorrectly positioned and sinks into the crevice.2 Sitting backwards on a chair and leaning forward to lift the arms and support the upper body may also be beneficial if the patient is still mobile.
- *Patients with single-sided lung dysfunction may benefit from lying on their side with the affected lung positioned downwards
- Optimising the patient’s immediate environment. 2 Depending on patient preference, this may include opening curtains and windows to allow light in and fresh air to circulate around the patient.6 Remind family/whānau to avoid smoking/vaping around the patient. A small fan directed towards the face may be beneficial for some patients as evidence has shown this can reduce the sensation of dyspnoea.4 Consider a dehumidifier as excessive humidity can worsen dyspnoea.2
- Interventions to reduce anxiety.2 For example, relaxation and distraction techniques, listening to music, spending time with family/whānau.6
- Consider the need for nicotine replacement therapy, e.g. patches, for patients with a history of smoking and who are at risk of withdrawal symptoms.10
- Complimentary or alternative medicines. The patient or their family/whānau may want to try traditional techniques and methods for symptom relief, e.g. Rongoā Māori, Ayurvedic or Chinese herbal medicines. These should be supported if they are unlikely to cause harm.
 Practice point: A person’s head is considered tapu (sacred) by Māori, and things that touch the head (e.g. facecloths and pillows) are also tapu by extension.8 If a pillow has been used to support another part of the body (especially the lower half of the body), it should not be reused to support the patient’s head.8 Different coloured pillowcases can be used to help observe this.
Practice point: A person’s head is considered tapu (sacred) by Māori, and things that touch the head (e.g. facecloths and pillows) are also tapu by extension.8 If a pillow has been used to support another part of the body (especially the lower half of the body), it should not be reused to support the patient’s head.8 Different coloured pillowcases can be used to help observe this.
Morphine is the most widely studied and extensively used medicine for the treatment of dyspnoea in patients with a terminal condition.9 The exact mechanism by which morphine alleviates dyspnoea is unknown, however, opioid receptors are located throughout the respiratory tract and are known to reduce respiratory drive; they may also reduce the sensation of dyspnoea.9 Anticipatory prescribing of “as needed” doses of morphine is recommended in patients without current dyspnoea (unapproved indication); if dyspnoea arises the “as needed” dose can be administered and then regular dosing initiated.6
Initiating opioids for dyspnoea
Opioid naïve patients. Initially low doses may be prescribed on an "as needed" basis, e.g. 1 – 5 mg of morphine, subcutaneously, every one to two hours, as needed (Figure 1).6 Exact dosing will be determined by the patient’s overall clinical condition.
Patients already taking opioids. Many patients in their last days of life will already be taking oral or transdermal opioids for other indications. Convert their daily oral opioid doses to a 24-hour dose for continuous subcutaneous infusion. A further dose for “breakthrough” symptoms can then be calculated by dividing their total 24-hour dose by six.6 This “breakthrough” dose should be prescribed every hour, as needed and also delivered subcutaneously.6 N.B. Morphine is normally prescribed two to four hourly, however, in an end of life care setting, doses may be given hourly if required.10 For patients already using fentanyl patches, see: “Morphine may not be appropriate for all patients”.
Review patient response. Ideally, patients should be reassessed within six hours of morphine initiation to review their response to treatment, check for adverse effects and if required, escalate the dose.6 If there has been mild improvement in symptoms but the patient remains distressed by their dyspnoea, escalate the daily morphine dose by the “total breakthrough” dose in the last 24 hours.6 A new “breakthrough” dose will now need to be calculated.6
Morphine may not be appropriate for all patients
If a patient is unable to take morphine due to an allergy, severe renal impairment or intolerable adverse effects, an alternative strong opioid can be trialled, however, oxycodone and fentanyl have not been as extensively studied for the management of dyspnoea as morphine.2
 Morphine in patients with renal impairment
Morphine in patients with renal impairment
In patients with renal impairment, the active metabolites of morphine (or oxycodone) can accumulate resulting in opioid toxicity.1, 11 Subcutaneous fentanyl is usually the most appropriate option for patients with significant renal impairment (i.e. eGFR < 30 mL/min/1.73 m2),6 however, dosing and administration is more complicated than morphine, and contraindications for treatment in this setting become less absolute. Expert opinion is that in patients with severe renal impairment who are imminently dying, small doses of subcutaneous morphine, e.g. 2.5 mg, every six hours, as needed, can be used effectively for dyspnoea with minimal risk of adverse effects.
Oxycodone (unapproved indication) administered subcutaneously is considered approximately dose-equivalent* to subcutaneous morphine,12 and can be initiated at a similar dose for dyspnoea. Patients already prescribed oral oxycodone for other indications should have their current dose converted to a 24-hour dose for continuous subcutaneous infusion and prescribed further “breakthrough” doses of oxycodone (one-sixth of the total 24-hour dose), as needed (Figure 1).6
*This applies to both subcutaneous and intravenous routes (but not the oral route where the ratio of morphine to oxycodone is 1.5:1 or 2:1)12
Fentanyl (subcutaneous; unapproved indication) is usually considered first-line for patients with dyspnoea and significant renal impairment, i.e. an eGFR < 30 mL/min/1.73 m2 (Figure 2);6 however, dosing and administration is more complicated than morphine. Discussion with the local hospice or palliative care team may be required.
Prescribe 10 – 20 micrograms of subcutaneous fentanyl, every hour, as needed. Patients who are still experiencing dyspnoea 12 hours after the first fentanyl dose should be initiated on 100 – 300 micrograms fentanyl over 24 hours via continuous subcutaneous infusion. After 24 hours the patient should be reviewed; those who require more than three “breakthrough” doses in this period should have their 24-hour subcutaneous fentanyl dose increased by the total “breakthrough” dose.
 Practice point: Fentanyl patches take 72 hours to reach steady state and should not be initiated in patients who are opioid naïve.1 The lowest dose fentanyl patch (12.5 micrograms/hour) is equivalent to up to 60 mg of oral morphine.1 Patients already prescribed fentanyl patches can continue to use them.13
Practice point: Fentanyl patches take 72 hours to reach steady state and should not be initiated in patients who are opioid naïve.1 The lowest dose fentanyl patch (12.5 micrograms/hour) is equivalent to up to 60 mg of oral morphine.1 Patients already prescribed fentanyl patches can continue to use them.13
Benzodiazepines for dyspnoea
There is no evidence that benzodiazepines reduce dyspnoea directly,14 however, they are effective at reducing the associated feelings of panic and anxiety, which in turn may reduce the sensation of dyspnoea.15 Benzodiazepines should be the next step if non-pharmacological interventions and morphine have not sufficiently relieved symptoms.14 They can also be considered in patients with reduced renal function,6 if higher doses of morphine are not appropriate.
Benzodiazepines are usually used in combination with opioids, however, a degree of sedation is inevitable. Doses should be titrated to effect and proportional to the severity of symptoms and patient-related goals.
The recommended dose is 5 – 15 mg midazolam over a 24-hour period via continuous subcutaneous infusion.6 Further subcutaneous doses of 2.5 mg midazolam can be administered hourly, as needed.6
Oxygen for patients with dyspnoea and hypoxaemia
Initiation of oxygen for patients in the last days of life is not commonly required.7 Oxygen treatment is only recommended for patients with dyspnoea and established hypoxaemia due to disease, i.e. oxygen saturation < 88%.16, 17 Consider potential adverse effects, e.g. oromucosal dryness, when deciding whether to initiate oxygen as these may outweigh any clinical benefit;18 discussion with the local hospice or palliative care team may help with decision making. Pharmacological management remains the priority when oxygen is initiated.
While there is not enough evidence to support oxygen treatment for patients with dyspnoea if hypoxaemia is not present,19 always consider individual patient goals. For example, oxygen therapy may be beneficial (alongside pharmacological measures) in patients who wish to remain at home in their last days of life, if there is clear progressive pathology, e.g. pleural or pericardial infusion.
Patient eligibility for home oxygen is determined by local criteria/policies and in some areas may require authorisation by a respiratory clinician.
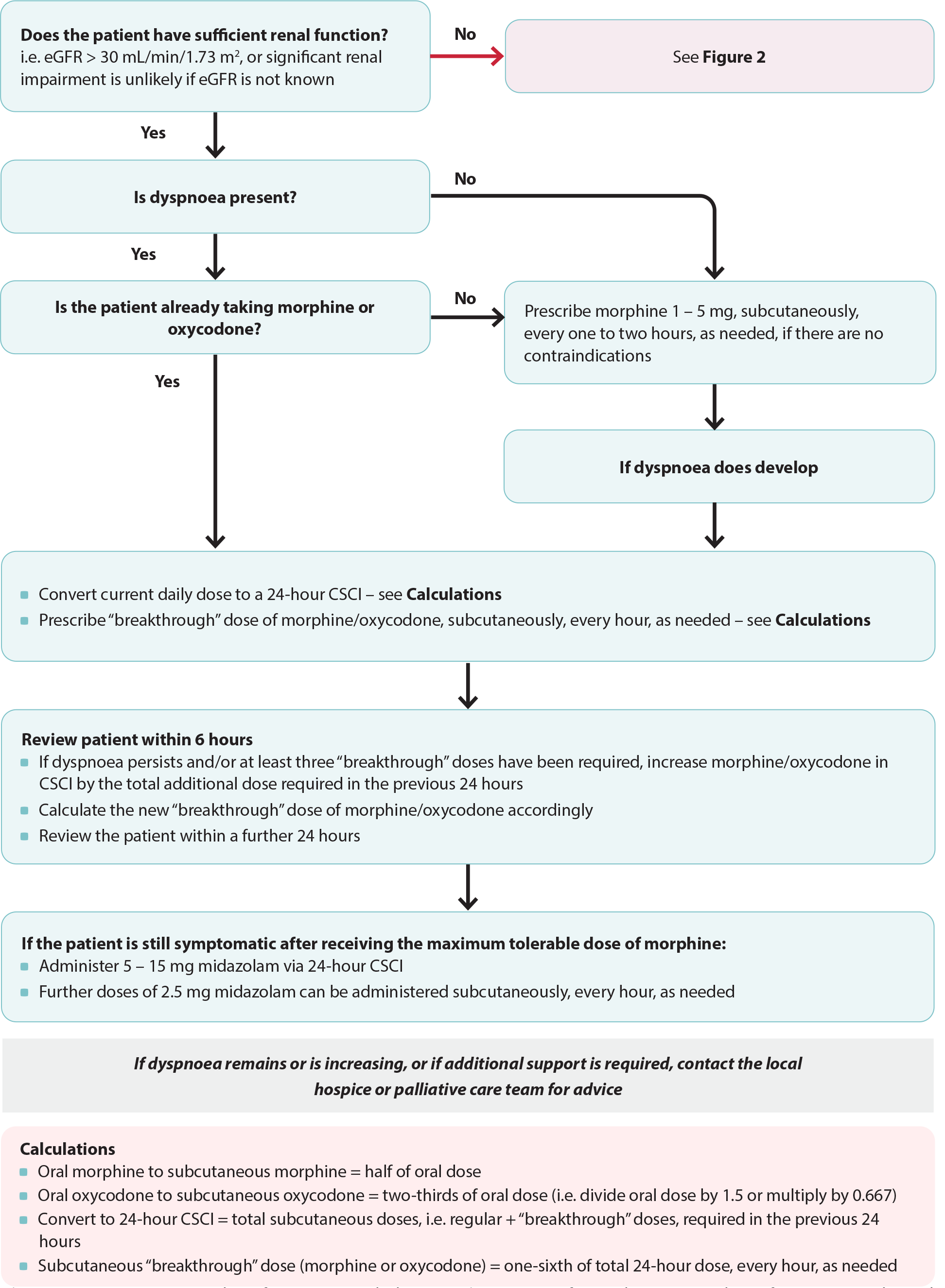
Figure 1. Anticipatory prescribing for patients with dyspnoea (see Figure 2 for an alternative pathway for patients with renal dysfunction). Adapted from South Island Palliative Care Workstream, 2020.6
CSCI = continuous subcutaneous infusion
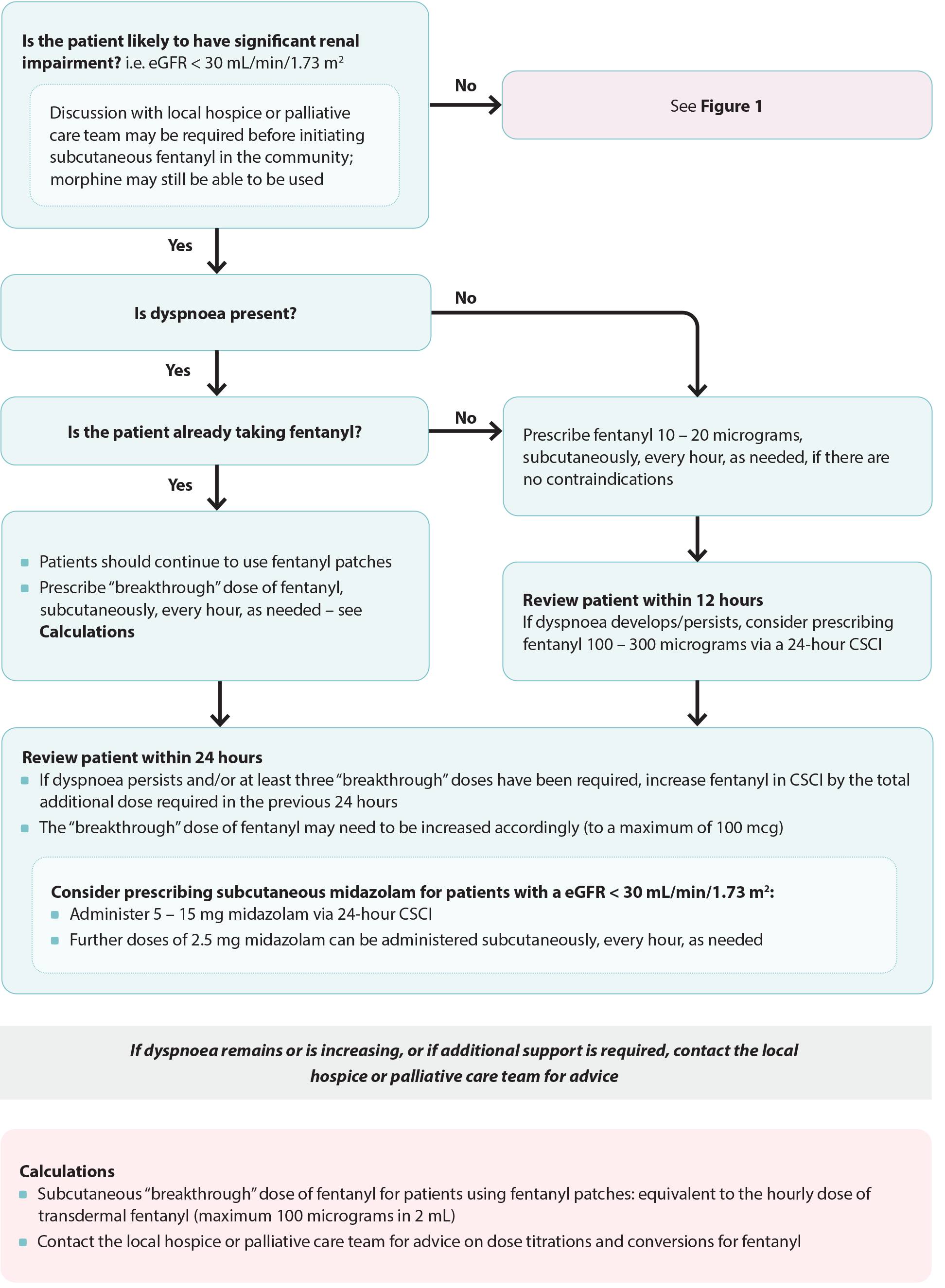
Figure 2. Anticipatory prescribing for patients with dyspnoea and reduced renal function. Adapted from South Island Palliative Care Workstream, 2020.6
CSCI = continuous subcutaneous infusion
Acknowledgement
Thank you to the following experts for review of this article:
- Dr Kate Grundy, Palliative Medicine Physician, Clinical
Director of Palliative Care, Christchurch Hospital Palliative
Care Service and Clinical Lecturer, Christchurch School of
Medicine
- Vicki Telford, Clinical Nurse Specialist, Nurse Maude
Hospice Palliative Care Service, Christchurch
- Dr Helen Atkinson, General Practitioner and Medical
Officer, Harbour Hospice
- Dr Robert Odlin, General Practitioner, Orewa Medical
Centre
- Fraser Watson, Extended Care Paramedic Clinical Lead,
Hato Hone St John
N.B. Expert reviewers do not write the articles and are not responsible for the final content.
bpacnz retains editorial oversight of all content.
This resource is the subject of copyright which is owned by bpacnz.
You may access it, but you may not reproduce it or any part of it except in the limited situations described in the
terms of use on our website.
Article supported by Te Aho o Te Kahu, Cancer Control Agency.

References
- MacLeod R, Macfarlane S. The palliative care handbook. 9th ed. 2019. Available from: https://www.hospice.org.nz/wp-content/uploads/2019/03/Palliative-Care-Handbook.pdf (Accessed September, 2023).
- Ministry of Health – Manatū Hauora. Te Ara Whakapiri Toolkit: care in the last days of life. 2017. Available from: https://www.health.govt.nz/system/files/documents/publications/te-ara-whakapiri-toolkit-apr17.pdf (Accessed September, 2023).
- Kamal AH, Maguire JM, Wheeler JL, et al. Dyspnea review for the palliative care professional: assessment, burdens, and etiologies. Journal of Palliative Medicine 2011;14:1167–72. https://doi.org/10.1089/jpm.2011.0109
- Azhar A, Hui D. Management of physical symptoms in patients with advanced cancer during the last weeks and days of life. Cancer Res Treat 2022;54:661–70. https://doi.org/10.4143/crt.2022.143
- Shellenberger RA, Balakrishnan B, Avula S, et al. Diagnostic value of the physical examination in patients with dyspnea. CCJM 2017;84:943–50. https://doi.org/10.3949/ccjm.84a.16127
- South Island Palliative Care Workstream. Te Ara Whakapiri Symptom management in the last days of life. 2020. Available from: https://www.sialliance.health.nz/wp-content/uploads/Symptom-management-in-the-last-days-of-life_SIAPO.pdf (Accessed September, 2023).
- Shin J, Chang YJ, Park S-J, et al. Clinical practice guideline for care in the last days of life. Korean J Hosp Palliat Care 2020;23:103–13. https://doi.org/10.14475/kjhpc.2020.23.3.103
- Medical Council of New Zealand. Best health outcomes for Maori: practice implications. 2006. Available from: https://www.indigenouspsych.org/Resources/Best_Health_Outcomes_for_Maori.pdf (Accessed September, 2023).
- Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database of Systematic Reviews 2016;2019. https://doi.org/10.1002/14651858.CD011008.pub2
- Waitemata District Health Board. Palliative care: oral morphine initiation and dose titration guide for opioid naïve patients. 2014. Available from: http://www.saferx.co.nz/assets/Documents/388ffa9f0c/Morphine_titration_guide.pdf (Accessed September, 2023).
- NHS Scotland. Scottish palliative care guidelines - Morphine. 2021. Available from: https://www.palliativecareguidelines.scot.nhs.uk/guidelines/medicine-information-sheets/morphine.aspx (Accessed September, 2023).
- New Zealand Formulary (NZF). NZF v135. Available from: www.nzf.org.nz (Accessed September, 2023).
- NHS Scotland. Scottish palliative care guidelines - Fentanyl patches. 2021. Available from: https://www.palliativecareguidelines.scot.nhs.uk/guidelines/medicine-information-sheets/fentanyl-patches.aspx (Accessed September, 2023).
- Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database of Systematic Reviews 2016;: CD007354. https://doi.org/10.1002/14651858.CD007354.pub3
- Pisani L, Hill NS, Pacilli AMG, et al. Management of dyspnea in the terminally ill. Chest 2018;154:925–34. https://doi.org/10.1016/j.chest.2018.04.003
- Australian and New Zealand Society of Palliative Medicine & the Australasian Chapter of Palliative Medicine. Tests, treatments and procedures health professionals and consumers should question. 2021. Available from: https://www.hqsc.govt.nz/assets/Resource-library/Choosing-Wisely/Publications-resources/CMC0003-Australian-and-New-Zealand-Society-of-Palliative-Medicine-1.pdf (Accessed September, 2023).
- Barnett A, Beasley R, Buchan C, et al. Thoracic Society of Australia and New Zealand position statement on acute oxygen use in adults: ‘Swimming between the flags’. Respirology 2022;27:262–76. https://doi.org/10.1111/resp.14218
- Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. International Journal of Chronic Obstructive Pulmonary Disease 2008;3:11–29. https://doi.org/10.2147/copd.s698
- Hasegawa T, Ochi T, Goya S, et al. Efficacy of supplemental oxygen for dyspnea relief in patients with advanced progressive illness: a systematic review and meta-analysis. Respiratory Investigation 2023;61:418–37. https://doi.org/10.1016/j.resinv.2023.03.005





