Atrial fibrillation and flutter in primary care
Atrial fibrillation is under-diagnosed and under-treated
| * We have used the abbreviation AF to mean atrial fibrillation or flutter because the principles of
antithrombotic therapy and rate control are the same for atrial fibrillation and atrial flutter. However there are some
important differences. For example, achieving rate control can be more difficult in flutter than fibrillation, choice
of agents for rhythm control are different and people with lone or predominant flutter should be considered for ablation
therapy. |
Atrial fibrillation or flutter (AF)* occurs in approximately 1% of the general population. The prevalence doubles with
each successive decade over the age of 50 years and it occurs in approximately 10% of people over the age of 80 years.
An estimated 30 to 40 thousand people in New Zealand have AF and about one-third of these are unaware of it. Most GPs
probably have some patients with undiagnosed AF.
People with AF are at increased risk of stroke, heart failure and other cardiovascular events. AF is associated with
doubling of mortality rates, mainly due to ischaemic stroke. Overall the risk of ischaemic stroke in people with AF is
approximately 5% per year but this risk is not evenly distributed across people with this arrhythmia. For people at high
risk, the benefits of warfarin to lower this risk, outweighs the risks of serious bleeding from warfarin use. Therefore
thromboembolic risk assessment is required for all people with AF.
Warfarin is generally considered to be underutilised in the management of AF; it appears that approximately one-third
of people with identified AF are taking warfarin. This underutilisation almost certainly results from a cautious approach
to avoiding the risks of major bleeding with warfarin. These risks have probably been overstated.
Many people with AF are prescribed digoxin for its beneficial effect of lowering the heart rate. However digoxin does
not control the heart rate during exercise. Its use as first-line therapy is limited to people who are unlikely to be
active or have overt heart failure.
The New Zealand Guidelines Group guideline �The management of people with atrial fibrillation and flutter� (NZGG, 2005)
makes recommendations, which if implemented can be expected to improve the primary care management of people with AF.
These recommendations form the basis for this article.
Table 1: People with atrial fibrillation
| Undiagnosed |
On Warfarin |
| Not on warfarin |
| Of the estimated 35,000 people in New Zealand with AF only two thirds are aware of it and only about
one-third of those with identified AF are on warfarin. |
Opportunistic screening recommended
Opportunistic screening of the radial pulse for irregularity can help to identify people with asymptomatic atrial fibrillation.
The diagnosis needs to be confirmed by ECG, which will also show the heart rate and may suggest underlying cardiac pathology.
Case finding is likely to be higher in older patients or those with cardiac or other conditions often associated with
AF. AF appears to occur in M?ori people at ages about ten years younger than the general population, probably related
to earlier onset of heart disease.
AF is often associated with:
- Cardiac conditions including hypertension
- Hyperthyroidism
- Alcohol excess
- Severe infection
- Pulmonary pathology
AF symptoms
AF results in asynchronous atrial contractions, which reduce cardiac efficiency, and an irregular and usually rapid
ventricular rate, which reduces diastolic filling time and coronary perfusion. Most people with AF, but not all, get symptoms
from these effects. The most common are palpitations, breathlessness, fatigue, light-headedness and chest discomfort but
at times AF can contribute to acute heart failure, myocardial ischaemia and hypotension.
When AF is paroxysmal it may not be present on a standard ECG. Some form of continuous monitoring, such as Holter monitoring
or event recording, may be required for people with intermittent symptoms suggestive of paroxysmal AF.
Initial assessment for people with AF
Appropriate initial assessment for all people with AF includes checking for the common causes of AF discussed above,
performing a thromboembolic risk assessment and doing any pre-treatment checks necessary before starting particular medications.
Apart from history, examination and ECG the assessment would usually include:
- Transthoracic echocardiogram
- CBC, TSH, renal function, LFTs, INR
- Thromboembolic risk assessment
Transthoracic echocardiography is performed to identify any underlying structural heart disease, which may need further
evaluation and information on disorders such as left ventricular hypertrophy, which may impact on thromboembolic risk
assessment. When there is likely to be delay in obtaining this examination warfarin therapy does not need to be delayed
for people who already meet the criteria for a strategy of rate control and warfarin therapy. Echocardiography is required
before a rhythm control strategy is instituted.
Other investigations may also be clinically indicated from this initial assessment.
Antithrombotic therapy and control of rate is the most appropriate strategy for most people with AF. The focus of AF
management is to control symptoms and reduce the risk of serious complications such as stroke or heart failure, as well
as managing associated pathology. The major components of AF management are antithrombotic therapy, to reduce the risk
of stroke, and rate or rhythm control, to reduce haemodynamic disturbance. Antithrombotic therapy and control of rate
is the most appropriate strategy for most people with AF.
Choosing between warfarin and aspirin for antithrombotic therapy in AF
Anticoagulation therapy with warfarin reduces the risk of stroke by approximately two thirds whilst aspirin reduces
it by one fifth. There is no difference in stroke risk between paroxysmal, persistent or permanent AF. Therapy should
be based on absolute stroke risk rather than current rhythm. The greater risk reduction by warfarin over aspirin must
be balanced against the increased risk of serious bleeding. In the average population this is approximately 1% per year.
The risk:benefit ratio for warfarin is most advantageous for people with a high absolute risk of stroke
The risk:benefit ratio for warfarin is most advantageous for people with a high absolute risk of stroke (>15% five year
stroke risk). Table 2 shows that when people with a 15% five-year stroke risk receive warfarin therapy there is a significant
decrease in stroke incidence. This stroke reduction is matched by an increase in major bleeding. However, although some
of this major bleeding will be intracranial haemorrhage, most will be GI or GU bleeding. At stroke risks of greater than
15% there is a greater absolute risk reduction in stroke, without a matching increase in major bleeding, which remains
at 10%.
Risk:benefit of antithrombotic therapy
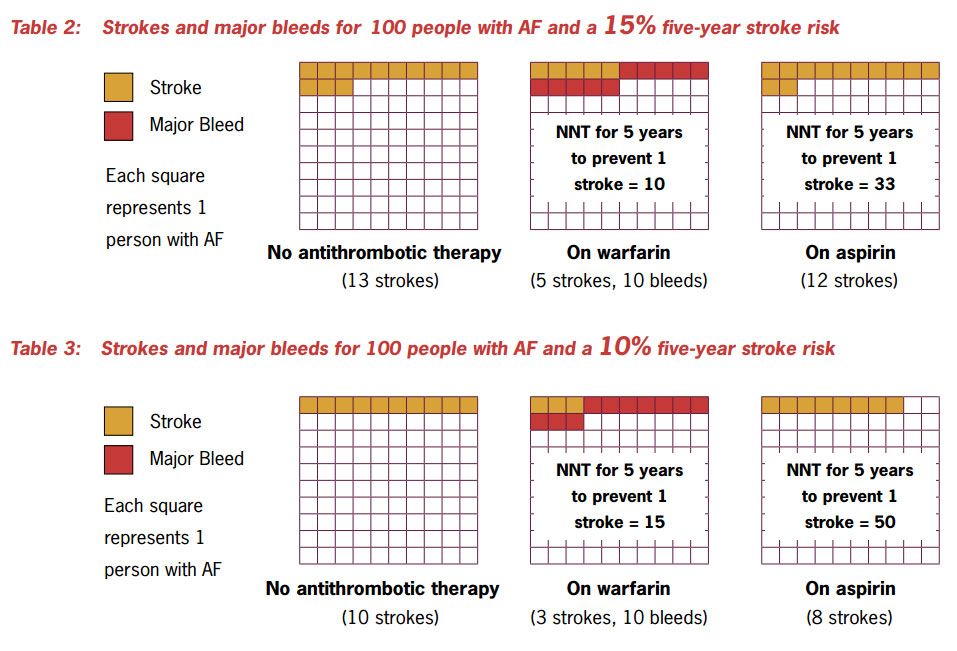
At low levels of stroke risk (<10% five year stroke risk) the risks of warfarin outweigh its benefits and aspirin
is a more appropriate choice. See Table 3. At intermediate levels of risk the benefits are not so clear-cut.
Thromboembolic risk assessment necessary to choose therapy
It can be seen from Table 4 that in order to choose the appropriate antithrombotic therapy a thromboembolic risk assessment
is necessary. Table 5 indicates factors which are useful in this assessment.
Table 4: Choice of therapy guided by thromboembolic risk
Get a PDF of tables 4 & 5 here
| Thromboembolic risk - five years |
| High risk of stroke (≥15%) |
Warfarin usually advantageous |
| Intermediate risk (10-14%) |
Discuss patient preferences |
| Low risk of stroke (<10%) |
Aspirin usually preferred |
| Very low risk of stroke |
Antithrombotic therapy not indicated |
Table 5: Risk factors for thromboembolic risk assessment
| High risk factors |
- Significant valvular heart disease (including mitral stenosis and prosthetic valves)
- Previous stroke, TIA or pulmonary embolus
- Heart failure or significant LV dysfunction
|
People with AF and one or more of these factors are at high risk of stroke |
| Medium risk factors |
- Woman >64 years
- Man >74 years
- Hypertension
- Diabetes mellitus
|
People with AF and two or more of these factors are at high risk of stroke.
People with AF and only one of these factors are at intermediate risk of stroke |
| Very low risk of stroke |
- Under 60 years with lone AF and no identified underlying cause, no hypertension and no clinical or echocardiographic
evidence of heart disease
|
Very low risk of stroke and unlikely to benefit from antithrombotic therapy |
Anticoagulation with warfarin requires a systematic practice-wide approach to INR monitoring.
 Warfarin
therapy for stroke prevention in AF can usually be initiated and maintained in primary care. This is discussed in bpacnz
publication ‘INR
Testing’. An audit for your practice’s system for monitoring INRs can be obtained by emailing bpacnz or by clicking here
for a ‘PDF’ version.
Warfarin
therapy for stroke prevention in AF can usually be initiated and maintained in primary care. This is discussed in bpacnz
publication ‘INR
Testing’. An audit for your practice’s system for monitoring INRs can be obtained by emailing bpacnz or by clicking here
for a ‘PDF’ version.
It is not always safe to give people warfarin even if their stroke risk is high; however the dangers of warfarin therapy
are often overstated. Discussion of when to exclude people from warfarin therapy can be found here.
Rate control is usually preferred to rhythm control to reduce the haemodynamic disturbance of AF
Rate control is the recommended strategy for most, but not all people with AF. Compared to rhythm control it reduces
morbidity and future hospitalisations and there appears to be no difference in the effects on mortality. However some
people will benefit from control of rhythm.
First identify people who are likely to benefit from rhythm control
People with any of the following are likely to benefit from pharmacological or non-pharmacological rhythm control, which
is conversion to and maintenance of sinus rhythm:
- Significant haemodynamic compromise, angina, MI or acute pulmonary oedema as a result of rapid AF; immediate cardioversion
is usually indicated, and warrants immediate referral to hospital
- Wolff Parkinson White Syndrome (WPW) with AF can lead to sudden death and warrants immediate referral to hospital
Unacceptable arrhythmia related symptoms despite satisfactory rate control
- Possibly young patients without structural heart disease (lone AF)
If a rhythm control strategy is chosen for people who are not yet anticoagulated, they should be cardioverted within
48 hours of onset of AF. If this deadline cannot be achieved, cardioversion will need to be delayed until an INR ≥2
has been achieved for four weeks or transoesophageal echocardiogram (TOE) has excluded atrial thrombi. The patients must
be fully anticogulated at least four weeks post cardioversion even if TOE shows no thrombus.
The pharmacological and non-pharmacological techniques used to cardiovert patients in AF to sinus rhythm are not available
to New Zealand GPs. Specialist referral is required. Antiarrhythmic therapy for maintenance of sinus rhythm should generally
be guided by physicians or cardiologists because of potential serious complications of new or more frequent occurrence
of pre-existing arrhythmias and non-cardiac side effects.
Rate control is recommended for most people with AF
Good rate control in AF can not only control symptoms but also improve outcomes by decreasing adverse results of AF
such as left ventricular dysfunction and cardiomyopathy.
Measures of good control of ventricular rate in AF are ongoing maintenance of:
- Resting ventricular rate of 60 - 80 bpm
- Ventricular rate during moderate exercise (6 minute gentle walk) 90 - 115 bpm
- No symptomatic palpitations or dyspnoea during exercise
These measures need to be reviewed regularly. Ventricular rate cannot be measured at the wrist as the radial pulse rate
significantly underestimates ventricular rate because of intermittent short coupling intervals. Ventricular rate must
be measured either at the apex or from the ECG.
In primary care apical pulse measurement immediately following a six minute walk is optimal and is validated in clinical
trials. If there are clinical concerns ventricular rate can be measured by Holter monitor (target 24 hour average <100
bpm) or exercise heart rate with a treadmill ECG.
Choice of rate control agent is guided by comorbidities
Table 6 lists rate-control agents in order of preference taking into account other conditions
that may be present. A combination of these may be required to achieve good control. People who only get occasional paroxysmal
AF, may be reluctant to take ongoing rate control medication for their intermittent problem, and can use medication as
needed to control symptoms. However there is little evidence for the benefit of this approach and most people with paroxysmal
AF are still likely to benefit from appropriate antithrombotic therapy.
Table 7 gives additional information about the use of ratecontrol agents.
Table 6: Choice of rate-control agent
Get a PDF of this and table 7 here
| Comorbidity |
First-line |
Second-line |
Less effective or desirable |
| No heart disease |
Beta-blockers*
OR
Calcium channel blockers** |
|
Digoxin***
(can be first-line in people unlikely to be active) |
| Hypertension |
Beta-blockers*
OR
Calcium channel blockers** |
|
Digoxin*** |
| Ischaemic heart disease |
Beta-blockers* |
Calcium channel blockers**
OR
Digoxin*** |
Ablation and pacing |
| Congestive Heart Failure |
Digoxin in overt heart failure
Carvedilol or metoprolol in stable heart failure |
Beta-blockers* (excluding carvedilol and metoprolol) OR Diltiazem |
Amiodarone
Ablation and pacing should be considered |
| COPD |
Calcium channel blockers** |
Beta-blockers*
(unless there is reversible bronchospasm) |
Digoxin*** |
* excluding sotalol
** diltiazem or verapamil
*** as monotherapy (can be used in combination with other rate-control agents) |
Table 7: Oral pharmacological agents for rate control in people with atrial fibrillation/atrial
flutter
Get a PDF of this and table 6 here
| Drug |
Oral loading dose |
Onset of action |
Commonly used oral maintenance doses |
Adverse effects |
Comments |
| Beta-blockers |
| Atenolol |
N/A |
2-3 hr |
25-50 mg |
Hypotension, heart block, bradychardia, asthma, heart failure |
In people with heart failure lower doses may be advisable (negative inotropic
effect) |
| Carvedilol |
N/A |
60-90 min |
6.25-25 mg/bd |
| Metoprolol |
N/A |
4-6 hr |
23.75-200 mg/day* |
| Nadolol |
N/A |
3-4 hr |
20-80 mg/day |
| Propranolol |
N/A |
60-90 min |
80-240 mg/day |
| Calcium channel blockers |
| Diltiazem |
N/A |
1-4 hr |
120-360 mg/day |
Hypotension, heart block, heart failure |
In people with heart failure, lower doses may be advisable |
| Verapamil |
N/A |
1-2 hr |
120-360 mg/day |
Hypotension, heart block, heart failure, digoxin interaction |
In people with heart failure, lower doses may be advisable (negative inotropic effect) |
| Other |
Digoxin
|
0.5-1.0 mg |
2 hr |
0.0625-0.375 mg/day |
Digoxin toxicity, heart block, bradychardia |
First-line therapy only for people unlikely to be active (e.g, older people or infirm)
and for people with heart failure. Less effective in hyperadrenergic states |
Amiodarone
|
400-800 mg/day for 1 week
|
1-3 week |
200 mg/day |
Photosensitivity and other skin reactions, pulmonary toxicity, polyneuropathy, gastrointestinal
upset, bradychardia, hepatic toxicity, thyroid dysfunction, torsades de pointes (rare) |
Although there is fairly good evidence of efficacy, this is an agent of last resort
in this indication, due to its long-term toxicity |
* The controlled release presentation of metoprolol is most commonly used.
N/A = Not applicable
Oral therapy usually provides effective rate control in AF. Other interventions such as IV administration
of antiarrhythmic agents or atrioventricular nodal ablation and pacemaker implantation may be required. |
Most people with AF can take warfarin safely
Contraindications to warfarin therapy
The major contraindication to warfarin therapy is where risk of haemorrhage outweighs the benefits of anticoagulation
Table 8: Exclusion criteria used for trials of warfarin in AF.
- Significant Thrombocytopenia (platelet count <100x109/L)
- Unexplained anaemia (Hb <100g/L)
- Bleeding disorders
- Past intracranial or retinal haemorrhage
- GI or GU bleed in previous six months
- Previous severe bleeding on warfarin with INR in target range
- Recurrent unexplained syncope
- Uncontrolled hypertension
- Renal failure
- Alcoholism
- Expected poor compliance
- Pregnancy
|
In some people, for example those with a bleeding disorder the risk is obvious, for others the risk is less overt. The
NZGG gives a list of conditions, which have been used to exclude people from the trials of warfarin use in AF (Table 8).
We therefore cannot conclude that people with these conditions are likely to benefit from warfarin therapy.
Advance age is, in itself, not a contraindication to warfarin therapy
Not everyone with AF at high risk of stroke is able to take warfarin. However it is generally considered that warfarin
is under utilised for this indication in both primary care and hospital practice. For example many clinicians are reluctant
to prescribe warfarin for older people with AF because of fear of bleeding. It is true that bleeding risk from warfarin
does increase with age but paradoxically older people are at increased risk of stroke, and potentially have much to gain
from anticoagulation.
In primary care we are often concerned that participants in clinical trials are not like the people we see in our practices.
However, although it is true that people with AF in the community are older and have more comorbidities than participants
in the clinical trials of stroke prevention in AF, we can be reassured that reviews of the evidence confirm that stroke
and bleeding rates with AF are comparable between trial participants and those in the community.
The NZGG document ‘The management of people with atrial fibrillation and flutter’ presents a useful table
of contraindications to warfarin therapy because of bleeding risk in older people (Table 9). Reference to this table can
give us more confidence in the use of warfarin.
Warfarin is contraindicated in pregnancy
Warfarin is teratogenic and should not be used in pregnancy.
Table 9: Contraindications to warfarin therapy in older people
Get a PDF of table
9 here
| Absolute Contraindication |
Relative Contraindication |
No Contraindication |
| Bleeding diathesis |
Conventional NSAID use |
Predisposition to falls |
| Thrombocytopenia (<50 x 103/μL) |
Participation in activities with high risk of trauma |
Advanced age |
| Hypertension (untreated or poorly controlled, consistently >160/90) |
Unexplained anaemia |
NSAID plus PPI |
| Non-adherence to treatment & monitoring |
Dementia |
Coxib use |
| Previous intracranial or retinal bleed |
Multiple comorbidities |
Recent resolved PU bleeding (with H. pylori testing and treating) |
| Recent GI or GU bleeding |
Unexplained recurrent syncope |
Previous ischaemic stroke |
Stroke Risk Stratification
Get a PDF of the tables here
The Baseline Risk of Stroke in People with New-onset AF (and without prior TIA or stroke) from Framingham Data (5-year
stroke risk in %)
| People with AF and either significant valvular disease, prior stroke
or TIA are at VERY HIGH risk of stroke and don’t need risk stratification.
They should receive long-term warfarin unless contraindicated. |
| People with AF and either left ventricular dysfunction (LVEF ≤ 40%)
or a past episode of decompensated heart failure are at HIGH risk and should receive
long-term warfarin unless contraindicated. |
Choice of warfarin or aspirin depends on stroke risk*
| Stroke Risk |
% Risk |
Treatment |
| VERY HIGH |
≥ 20% |
Long-term anticoagulant treatment with adjusted dose of warfarin aiming
for an INR 2.5 (range 2.0 to 3.0) unless there are clear contraindications |
| HIGH |
15 - 19% |
| INTERMEDIATE |
10 - 14% |
Discuss the individual’s potential benefits, risks and preferences for or against
anticoagulant or aspirin treatment |
| LOW |
< 10% |
Commence aspirin (75 mg to 300 mg) after discussion |
Note: In people with a contraindication to warfarin, consider using aspirin (75 mg to 300 mg) after discussion.
* Even when risk of stroke is high careful consideration of contraindications is required before warfarin is
commenced.
5-year Stroke Risk (%)
| WOMEN |
| No-Diabetes |
|
Diabetes |
Systolic
Blood
Pressure
(mm Hg) |
180 |
23 |
AGE
≥ 75 |
37 |
| 160 |
20 |
34 |
| 140 |
18 |
31 |
| 120 |
16 |
28 |
|
|
|
|
|
Systolic
Blood
Pressure
(mm Hg) |
180 |
18 |
AGE
65-74 |
29 |
| 160 |
16 |
27 |
| 140 |
14 |
24 |
| 120 |
13 |
21 |
|
|
|
|
|
Systolic
Blood
Pressure
(mm Hg) |
180 |
13 |
AGE
< 65 |
22 |
| 160 |
12 |
20 |
| 140 |
11 |
17 |
| 120 |
10 |
16 |
|
|
| MEN |
| No-Diabetes |
|
Diabetes |
Systolic
Blood
Pressure
(mm Hg) |
180 |
13 |
AGE
≥ 75 |
22 |
| 160 |
11 |
19 |
| 140 |
10 |
17 |
| 120 |
9 |
15 |
|
|
|
|
|
Systolic
Blood
Pressure
(mm Hg) |
180 |
10 |
AGE
65-74 |
17 |
| 160 |
9 |
15 |
| 140 |
8 |
13 |
| 120 |
7 |
12 |
|
|
|
|
|
Systolic
Blood
Pressure
(mm Hg) |
180 |
7 |
AGE
< 65 |
13 |
| 160 |
6 |
11 |
| 140 |
6 |
10 |
| 120 |
5 |
9 |
|
Expert Review: Dr David Heaven. Consultant Cardiologist, Middlemore Hospital