Published: 29th September 2023 | Updated: 12th November 2024
What's changed?
Updated: 12th November 2024 General revision.
Updated: 5th October 2023 Information added on HPV testing results for people with immune deficiency
If you would like to know what changes were made when the article was updated please contact us
 HPV Primary Screening: an overview of the programme
HPV Primary Screening: an overview of the programme
Practice tip. If you change the gender of a patient who is biologically female in their clinical record, ensure they remain in the practice recall system for cervical screening and are enrolled with the National Cervical Screening Programme.
 Eligibility for funded cervical screening
Eligibility for funded cervical screening
Cervical screening is funded for:
- People with a cervix aged ≥ 30 years who are unscreened (never had cervical screening) or under-screened (no normal cytology test in the past five years [or three years if immune deficient] or no normal HPV test in the past seven years [or five years if immune deficient]). This includes people aged 70 – 74 years who are unscreened or under-screened.
- People with a cervix aged 25* – 69 years who are of Māori or Pacific ethnicity
- People with a cervix aged 25* – 69 years who are a Community Services Card holder
- Anyone requiring follow-up or surveillance; even if they were not eligible for funded screening for their initial test
* People with a cervix aged under 25 years who have already started cervical screening (prior to 2019, the start age was 20 years) and are of Māori or Pacific ethnicity or a Community Services Card holder are also eligible for funded cervical screening.
Click here for further guidance. N.B. Cervical screening is only funded if the patient is due for screening or if screening is due in no more than six months.
N.B. For people who are not eligible for funding, it is up to the practice to determine the co-payment they will charge for HPV testing.
 Conducting HPV testing
Conducting HPV testing
What type of sample to take?
- HPV testing can be performed from one of the following samples:
- A vaginal swab taken by the patient (i.e. a self-test)
- A vaginal swab taken by a screen taker
- Liquid-based cytology (LBC) taken by a cervical sample taker. This sample type will only be tested for HPV initially (in routine screening). If HPV is detected, reflex cytology will then be performed.
- A LBC sample for both HPV and cytology testing is required for people:
- With symptoms (note specific symptoms on the laboratory form)
- Needing a test of cure (note on the laboratory form that a co-test is required for a test of cure)
- Needing a co-test for follow-up, e.g. people with completely excised HPV negative or HPV status unknown adenocarcinoma in situ (clinical details must be noted on the laboratory form)
- A LBC sample may be a pragmatic choice in the following scenarios so that if HPV is detected, a return visit is not required (as reflex cytology will be performed on the same sample):
- People with previous low-grade results who have not returned to regular screening
- At 12 or 24 month follow-up after a “HPV Detected Type Other” result
- People with immune deficiency – this is because people with immune deficiency should be referred for colposcopy if HPV of any type is detected and where possible this should be informed by cytology results
- People who may find follow-up difficult, e.g. live rurally, unlikely or unable to return
- Some people may also prefer for a clinician (i.e. a cervical sample taker) to perform a speculum examination and take a LBC sample for their cervical screen
- Refer to the clinical practice guidelines for HPV testing and/or cytology recommendations in special clinical circumstances, e.g. people with cervical lesions, after hysterectomy, follow-up after colposcopy or treatments
How to conduct the test
- When a person is due for cervical screening, a consultation with a screen taker is required to discuss the test, including which sample collection method is most appropriate, and to gain informed consent for HPV testing (record in patient notes). Advise patients who choose a vaginal swab that they may need to return for further testing if HPV is detected.
- HPV self-testing should usually be carried out at the practice. HPV is highly transmissible, so to reduce the risk of HPV cross contamination, provide a suitable space for self-testing where the person can wash their hands prior to and after testing and dispose of any rubbish. Provide self-swabbing instructions to patients; these can be downloaded or ordered from HealthEd or your laboratory provider if needed.
- At the discretion of the screen taker, a patient may do their HPV self-test at another location, e.g. their home, but must return the sample to the practice. It is the screen taker’s responsibility to follow up that this has occurred (the time required for this follow-up may be a factor in the decision to offer off-site testing).
When can you stop HPV testing?
- People can exit the cervical screening programme from age 65 years (or age 67 years if immune deficient) if they have a “HPV Not detected” result and are not in follow-up for abnormal cytology or histology
- People aged 70 – 74 years who have been unscreened or under-screened prior to age 70 years should have a HPV test and can exit the programme once HPV is not detected. This includes people transitioning from the cytology-based programme without two consecutive normal cytology results after the age of 62 years, and people who have already transitioned to the new screening programme who have not had a negative HPV test in the five years prior to age 70 (or three years prior to age 70 if immune deficient).
 Facts and tips about HPV testing
Facts and tips about HPV testing
- HPV testing can be done during menstruation if necessary (although not preferable), but occasionally excessive blood on the swab may cause invalid results and a repeat test will be needed (the repeat test would be funded)
- HPV testing (including from a LBC sample if needed) can be done during pregnancy and postpartum. Patients should still be referred for colposcopy if required, but biopsy may be delayed.
- Vaginal oestrogen cream may be considered for vaginal atrophy if cervical screening is likely to be painful, but a swab cannot be taken if the sample will be obscured by cream
- Testing for STIs must be done on a different swab to the one being tested for HPV
- Recent HPV vaccination does not affect the results of HPV testing
- HPV can be latent and reactivate, so can be detected even if someone is not currently sexually active but has been in the past
 Samples and results
Samples and results
- Dry swabs are used for HPV testing (red top; wet swabs were phased out by September, 2024).
- Samples can be stored at room temperature before transport; although samples should be sent to the laboratory as soon as possible, swabs may be able to be processed up to one month after sampling.
- Results will either be: HPV Not detected, HPV Detected Type 16, HPV Detected Type 18, HPV Detected Type Other (some laboratories will say which type[s]), Invalid (sample processed but no result) or Unsuitable for analysis (e.g. not processed because the sample was leaking)
- The result report will include an overall recommendation. If HPV testing was facilitated by a HPV screen taker or HPV screening kaimahi, the overseeing clinician (the “responsible clinician”) is responsible for the management of the patient’s results, e.g. arranging appropriate referrals, follow-up and recalls.
 HPV testing results in brief
HPV testing results in brief
- If HPV Type 16 or 18 is detected (approximately 2.5% of results), refer for a colposcopy
- If HPV Type Other is detected (approximately 7.5% of results) on a vaginal swab, a return visit for a cervical LBC sample to be taken by a clinician (i.e. a cervical sample taker) with a speculum is required. If the HPV test was initially performed on a cervical sample, cytology will automatically be reported, and a return visit is not required. Next steps are dependent on cytology results – see Figure 1 for details.
- If HPV of any type is detected in a person who is immune deficient, they should be referred for colposcopy, informed by cytology test results where possible (but this should not delay referral)
- If HPV is not detected (approximately 90% of results), no further action is required – place a recall for screening in five years (or three years if immune deficient)
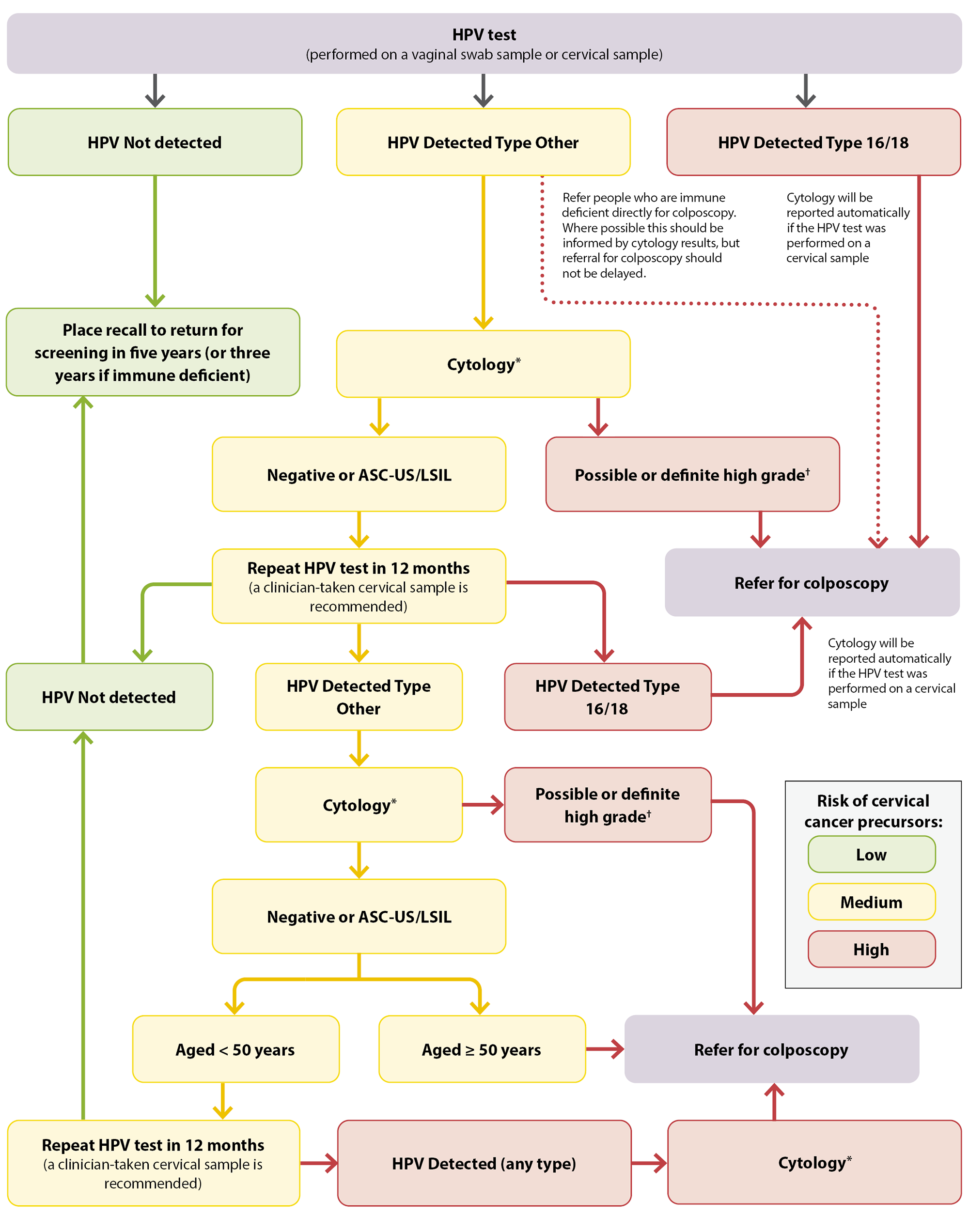
*Cytology cannot be performed from a vaginal swab sample. If the HPV test was conducted from a vaginal swab sample, a return visit is required for a clinician-taken taken (i.e. by a cervical sample taker) cervical LBC sample with speculum examination.
†Possible or definite high-grade cytology includes ASC-H, HSIL, SCC, atypical glandular cells, adenocarcinoma and AIS
Figure 1. HPV testing pathway for females who are asymptomatic in New Zealand. Adapted from Clinical Practice Guidelines for Cervical Screening in Aotearoa New Zealand, 2023.
ASC-US = atypical squamous cells of undetermined significance; ASC-H = atypical squamous cells of undetermined significance cannot exclude HSIL; HSIL = high-grade squamous intraepithelial lesion; LSIL = low-grade squamous intraepithelial lesion; SCC = squamous cell carcinoma; AIS = adenocarcinoma in situ
 For further information, see:
For further information, see:

