People who live, work or undertake recreational activities in a rural, agricultural or horticultural setting, are potentially
exposed to a large number of infectious pathogens that can cause disease. Individually, most of these infections are
rare, but the possibility of a rurally-acquired infection should be considered in symptomatic patients who have been
exposed to this setting.
Many infections that were once prevalent in rural New Zealand have now been eliminated, e.g. hydatid parasites and
brucellosis. However, some infections, e.g. leptospirosis, orf and Listeria, are still occasionally seen in rural communities.
Leptospirosis, campylobacter enterocolitis, salmonella enterocolitis, cryptosporidiosis and giardiasis are
the most common rurally-acquired infections in New Zealand; these have been covered in previous articles in the rural
infections series.
To round up the list of other rural infections, we have categorised them by their primary risk factors, which are:
- Consumption of unprocessed foods and untreated water
- Exposure to animals
- Exposure to plants or soil
N.B. Many of these infections have more than one contributing cause, and some are not unique to the rural environment.
Infections acquired via consumption of unprocessed foods or untreated water
Many people living in a rural community do not have access to a reticulated water supply, and collect and store their
own water for household use. A rural lifestyle also often involves raising, growing and gathering food, e.g. raw milk,
home-butchered or recreationally-caught meat and seafood. These practices are all associated with an increased risk of
infectious diseases.
Drinking unpasteurised (raw) milk
Drinking milk “straight from the cow” is a way of life for many people living or working on a farm. The consumption
of raw milk products is also gaining popularity in the wider community. However, although regarded as “wholesome” or
“healthy”, drinking raw milk actually increases a person’s risk of illness.
Milk from cows, goats and sheep can be contaminated with bacteria, such as Campylobacter jejuni, Escherichia
coli, Listeria monocytogenes, Mycobacterium bovis, Salmonella enteritidis, Shigella
spp. and Yersinia enterocolitica. Pathogens can pass into milk directly via an infection in the animal,
e.g. mastitis in the udder, or indirectly from the farm environment during the milking process, e.g. faecal contamination.1 Commercially
produced milk is pasteurised to destroy these bacteria. Pasteurisation is a heat treatment process which usually involves
milk being rapidly heated to 72°C for 15 seconds.
There have been several small outbreaks of infectious diarrhoea associated with raw milk consumption in New Zealand
in recent years.1 The Ministry for Primary Industries monitors dairy products in New Zealand; an ongoing
survey has found Listeria monocytogenes, Shiga-toxin producing E.coli and Campylobacter jejuni in
raw milk.1 In the United States, the Centers for Disease Control and Prevention (CDC) states that “the consumption
of non-pasteurised dairy products cannot be considered safe under any circumstances”.2
Facts about pasteurised milk:1, 3
- Pasteurisation is a highly reliable method for eliminating pathogens in milk
- Pasteurisation has a minimal effect on the fat and protein composition of milk
- Pasteurisation does not affect mineral content, stability or gastric absorption of milk
- Riboflavin, vitamin B6 and B12 are reasonably heat stable so remain in pasteurised milk at high levels
- Pasteurisation reduces the vitamin C content in milk by approximately 10%, however, milk is not a significant dietary
source of vitamin C
- Some enzymes in milk are inactivated during the pasteurisation process but these are not thought to be important
for human health
It is recommended that:1
- Raw milk products should not be consumed by young children, elderly people, pregnant women or people who are immunocompromised
- If raw milk is consumed, ensure it is from a source where good hygiene practices are adhered to during milking and
storage (this reduces, but does not eliminate, the risk of contamination)
- Refrigerate raw milk at ≤ 4 ° C (this will not eliminate Listeria– see below)
- Discard raw milk if it has been at room temperature for more than two hours
- If diarrhoea develops after ingestion of raw milk, consider the possibility of an infectious pathogen as the cause
 For further information on Salmonella, Campylobacter and E.coli,
which can all be contaminants in unpasteurised milk, see: “Rural infections
series: Investigating and managing people with diarrhoea”, Best Tests (Feb, 2014). For information on Listeria,
also a milk contaminant, see below.
For further information on Salmonella, Campylobacter and E.coli,
which can all be contaminants in unpasteurised milk, see: “Rural infections
series: Investigating and managing people with diarrhoea”, Best Tests (Feb, 2014). For information on Listeria,
also a milk contaminant, see below.
 A focus on Listeria
A focus on Listeria
Listeria monocytogenes is a foodborne pathogen found in unpasteurised milk or unpasteurised milk products
(e.g. cheeses), and also in items such as processed meat products (e.g. salami, paté), cold pre-cooked meats, uncooked
seafood and raw vegetables, e.g. stored salads. L.monocytogenes can survive and multiply in food items at standard
refrigeration temperatures.4 People may also be exposed to L. monocytogenes via contact with potentially
infective farm material, such as aborted animal foetuses.4
Listeriosis, the illness caused by L.monocytogenes, is characterised by diarrhoea, nausea, vomiting, fever,
myalgia and fatigue, which typically resolve within one to three days.5 More severe complications, such as
the development of septicaemia or meningoencephalitis, are more likely to occur in vulnerable groups, such as pregnant
women, young infants, elderly adults and immunocompromised people. Listeriosis also causes risks to a pregnancy, including
miscarriage, premature labour and stillbirth. Listeria infection can be transferred to an infant during childbirth,
which can result in serious illness and death for the infant.6 There are approximately 25 notified cases
of listeriosis per year in New Zealand (see: “Listeriosis in New Zealand”).4
Listeriosis is often an unexpected diagnosis and rarely considered before being identified by laboratory testing. The
time between exposure and onset of symptoms is variable, with cases being reported between 1 – 70 days after exposure
to a contaminated food.4, 5 It is estimated that the median incubation period of Listeria is three
weeks.4 In practice it will be difficult to differentiate listeriosis from other diarrhoeal illnesses caused
by pathogens, such as Giardia, Salmonella, Campylobacter and E.coli. Laboratory investigation
is recommended in patients presenting with persistent diarrhoea and risk factors, e.g. exposure to a rural environment.
It can be important to ask people their occupation when they present with persistent diarrhoea as they may live in an
urban area, but work in a rural/agricultural environment.
If listeriosis is suspected (e.g. risk factors present and other likely pathogens have been ruled out), this can be
discussed with an Infectious Diseases Specialist or Clinical Microbiologist. The best test for L.monocytogenes is
blood culture; stool culture for Listeria is not routinely performed. Listeriosis is a notifiable disease and
cases (suspected or confirmed) must be notified to the local Medical Officer of Health.4
Management of listeriosis is usually in conjunction with an Infectious Diseases Specialist. Depending on the clinical
situation, patients with listeriosis may be managed at home if their signs and symptoms are mild. Patients with severe
signs and symptoms, and those most at risk of serious illness are managed in a hospital setting.4 Antibiotics
may be considered for symptomatic and asymptomatic people who are at high risk of complications (e.g. infants, pregnant
women, elderly adults, immunocompromised people), if they are known to have ingested a food implicated in an outbreak.5 Listeriosis
is treated with amoxicillin 1 g, three times daily, for 10 – 14 days.7 Co-trimoxazole is an alternative.5 Other
antibiotic choices for treatment may be considered in a hospital setting.6
Patients with listeriosis can remain infectious to others for several months after resolution of symptoms,4 however,
other than transplacental transmission (mother to foetus), there are few, if any, reported cases resulting from person
to person transmission.
Eating home-kill and recreational catch meat
In the rural community, many families will consume meat which has been butchered on the farm (home-kill) or hunted
(recreational catch). As these methods are not subject to any hygiene or safety regulations, there is a potential for
transmission of infectious diseases and toxicity via handling or ingestion of raw or under-cooked meat.
The main risks are:9
- Bacterial contamination from the animal via external wounds or contents of the gut or other infected organs
- Bacterial contamination from the environment, e.g. soil, grass, hunting knife
- Chemical contamination via the animal eating pest control poisons or carcasses of poisoned animals, or if transporting
the carcass in a vehicle used to carry chemicals, e.g. weed killer or fuel
Bacterial contaminants in home-kill and recreational catch meats include Salmonella (particularly birds), Campylobacter, Cryptosporidium (particularly
calves and lambs), Giardia and, rarely Trichinella (particularly pigs).
The Ministry for Primary Industries has guidelines on safe practices for home-kill meat. A consumer information brochure
can be found here: www.foodsafety.govt.nz/elibrary/consumer/Homekill-brochure-2012-web.pdf
 And further information found here: www.foodsmart.govt.nz/food-safety/hunting-collecting-fishing/
And further information found here: www.foodsmart.govt.nz/food-safety/hunting-collecting-fishing/
Listeriosis in New Zealand
In New Zealand, epidemiological data on listeriosis is collected by the Institute of Environmental Science and Research
Ltd (ESR). In 2012 (latest reported data) there were 25 notified cases of listeriosis (0.6 per 100 000 population).
Two of these cases were perinatal, which resulted in death of the foetus. Of the remaining cases most were in people
aged 50 years and over (21 cases). The majority (16 cases) also had an underlying co-morbidity, and four cases resulted
in death. The 25 notified cases were from nine DHBs, including five from Counties Manukau, five from Bay of Plenty and
four from Hawke’s Bay. There was one outbreak of listeriosis reported in 2012, linked to an infected ready-to-eat meat
product. The notification rate of listeriosis has been relatively stable over the past 15 years, following a peak of
cases in 1997 (0.9 per 100 000 population).8 It is likely that the actual rate of Listeria infection in
the population is higher than the notified rate, taking into account cases of sub-clinical or mild infection which are
not reported.
 A focus on Trichinella
A focus on Trichinella
Trichinella spiralis is a parasitic round worm that can be found in carnivorous animals, such as feral cats
and rats. There have been historical cases of infection among the domestic pig population in New Zealand, from pigs eating
carcasses and faeces of infected animals.10 However, the risk of T. spirialis in commercial piggeries
in New Zealand is now regarded as very low. Although extremely rare (only three notifications since 1988),8 infection
in humans can occur after ingestion of raw or under-cooked meat, i.e. pork, that contains encysted Trichinella larvae. Trichinella cannot
be transmitted from human to human.10
Trichinella can be destroyed by cooking meat until it reaches an internal temperature of ≥ 60°C for at least one minute,
or by freezing meat at ≤ –15°C (standard home freezer temperature) for at least 20 days. Curing, salting, smoking or
microwave cooking will not destroy Trichinella.10
Trichinellosis, the illness caused by T. spiralis, typically begins one to two days after ingestion of infected
meat, with general discomfort, abdominal pain and diarrhoea, lasting up to one week. Headache, fever and excessive sweating
may develop three to four days after ingestion. Further systemic features may occur within 8 – 15 days after ingestion
(range 5 – 45 days), such as facial oedema (usually periorbital), myalgia (most commonly affecting the trunk and limbs)
and severe weakness.10, 11 Patients with trichinellosis almost always have eosinophilia, which can persist
for several weeks to months.11 Other characteristic laboratory parameters include increased muscle enzymes
and increased total IgE. Differential diagnoses of trichinellosis include influenza, infectious diarrhoea and auto-immune
disease.11
Patients with suspected trichinellosis should be referred to an Infectious Diseases Specialist. Trichinellosis is confirmed
by a positive serological test or detection of larvae in muscle tissue biopsy. Treatment usually involves an anthelmintic
(e.g. mebendazole), analgesics, corticosteroids and supportive care.10, 11 Trichinellosis is a notifiable
disease so all cases, suspected or confirmed, should be notified to the local Medical Officer of Health.
 For further information about trichinellosis, see: FAO/WHO/OIE Guidelines for
the surveillance, management, prevention and control of trichinellosis. Available from: www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf
For further information about trichinellosis, see: FAO/WHO/OIE Guidelines for
the surveillance, management, prevention and control of trichinellosis. Available from: www.trichinellosis.org/uploads/FAO-WHO-OIE_Guidelines.pdf
Blastocystis: unknown role in infection
Blastocystis is a protozoan parasite which can be found in the gastrointestinal tract of many animals. Humans may
acquire infection from animals (particularly from cattle, pigs or birds) or from person-to-person oral-faecal contact.
Whether blastocystis is a cause of human disease is very uncertain. Some people found to have stool carriage of blastocystis
are asymptomatic, whereas some have diarrhoea and other gastrointestinal symptoms. It is thought that people who are
immunocompromised may be more susceptible to infection.16 Most mild symptomatic cases are self-limiting;
no specific treatment is required. However, in rare cases, gastrointestinal symptoms may be persistent. In these cases,
other pathogens, e.g. Giardia, should first be ruled out as a cause for the symptoms. If the symptoms appear
to be attributable to blastocystis, a course of metronidazole may be trialled. There has been mixed evidence of the
success of metronidazole in eradicating infection. If treatment with metronidazole has failed, or is contraindicated,
co-trimoxazole is a second-line option.16
Drinking tank water
Using collecting tanks or a natural ground water source for household water supply is common in rural communities in
New Zealand. Depending on the source of the collected water, e.g. stream, bore, rainwater, and the household storage
and filtering system used, contamination with infectious pathogens, heavy metals, trace elements and agricultural chemicals
is possible.
Human or animal waste is the most likely source of pathogenic micro-organisms in water supplies. Bacteria are also
found naturally in ground water and surface water.12
Drinking water may be contaminated from seepage from a septic tank, run-off from pastures, heavy rains causing overflowing
storm water, animal faeces (e.g. on a roof used for collecting rainwater), or improperly sealed storage tanks or wells.12
E.coli is one of the most common infectious pathogens in collected water and is used as a marker of faecal
contamination. Cryptosporidium, Giardia, Campylobacter, Salmonella and Shigella are
also common contaminants. Other micro-organisms found in water include helminths (thread worms, tape worms, nematodes)
and viruses, such as norovirus, rotaviruses and hepatitis A.12 These organisms can be found in faecal waste
of humans and animals (e.g. pigs, deer, sheep, cows, birds, possums) and also in raw milk.12 Most of these
pathogens cause gastrointestinal illness, and the most susceptible groups are young infants, elderly adults and people
who are immunocompromised. In some cases, people who have a prolonged exposure to a pathogen can develop immunity to
it. Therefore members of a household with a contaminated water supply may not display and signs and symptoms, but visitors
drinking the contaminated supply may become ill.12
If a patient presents with persistent diarrhoea and has a history of drinking from a tank water supply, testing for
infectious pathogens would be indicated. A faecal sample should be sent for culture (which tests for Campylobacter,
Salmonella, Yersinia, E.coli (VTEC) and Shigella) and antigen testing for Giardia and Cryptosporidium.
Note risk factors and relevant clinical details on the laboratory request form.
It is recommended that home water supplies are frequently tested for E.coli (also called faecal coliforms)
to monitor faecal contamination. At home kits are available or a sample can be sent to a commercial laboratory. An effective
water filtering system, e.g. a UV filter, will help to minimise risk.
 For further information on managing diarrhoea in a rural population, see: “Rural
infections series: Investigating and managing people with diarrhoea”, Best Tests (Feb, 2014).
For further information on managing diarrhoea in a rural population, see: “Rural
infections series: Investigating and managing people with diarrhoea”, Best Tests (Feb, 2014).
 For further information about drinking water guidelines, see: www.health.govt.nz/our-work/environmental-health/drinking-water
For further information about drinking water guidelines, see: www.health.govt.nz/our-work/environmental-health/drinking-water
Brucellosis: once endemic in New Zealand but now rare
Brucellosis is a granulomatous infectious disease caused by the ingestion of Brucella bacteria in raw milk
or meat from infected animals, or through contact with animal faeces or carcasses. Most cases of brucellosis in humans
are caused by B. melitensis, but B. abortus, B. suis and B. canis can also cause human illness.13
Brucellosis is a notifiable disease and between 1997 and 2012, 13 cases were reported in New Zealand.8 However,
these patients are presumed to have acquired the infection in other countries because the only Brucella species
that remains in New Zealand is B. ovis, which infects sheep, but is not pathogenic to humans. B. abortus was
once endemic in cattle in New Zealand but was eradicated by 1996; since then, there has been no evidence of locally-acquired
brucellosis in humans.14
People with brucellosis usually present with acute febrile illness, general malaise and respiratory tract symptoms.15 “Drenching”,
malodorous perspiration is a characteristic feature.13 Physical examination is generally nonspecific, however,
lymphadenopathy, hepatomegaly or splenomegaly may be present.13 If untreated, complications can include
granulomatous hepatitis, arthritis, spondylitis, anaemia, thrombocytopenia, meningitis, uveitis, optic neuritis, endocarditis
and neurological disorders collectively known as neurobrucellosis.13
Patients with suspected brucellosis should be referred to an Infectious Diseases Specialist. Laboratory confirmation
of brucellosis involves serological testing and culture.
Infections acquired via contact with animals
People with agricultural occupations, such as farmers, dairy workers and meat processors, and people who live on farms,
are exposed to a large number of infectious pathogens via contact with animals. For example, leptospirosis, which passes
from mammals, such as pigs and cattle, to humans, is the most common occupationally acquired infectious disease in New
Zealand.17
Animal-to-human contact is associated with respiratory infections, such as tuberculosis, and skin infections, such
as pox viruses, dermatophyte and erysipeloid infections and granulomas.
 For further information about leptospirosis, see: “Rural
infections series: Leptospirosis”, Best Tests (Nov, 2013).
For further information about leptospirosis, see: “Rural
infections series: Leptospirosis”, Best Tests (Nov, 2013).
Tuberculosis
In 2013 there were 278 cases of tuberculosis in New Zealand.18 Tuberculosis is now mostly seen in immigrants
and seasonal workers. Mycobacterium tuberculosis is the typical bacteria associated with tuberculosis, and is
transmitted from human-to-human. Atypical infections with other Mycobacterium species also occur. There are
multiple causative species, but the most common are M. kansasii and M. avium-intraceullulare, which
can be found in water, milk, bird excrement, soil and house dust. Atypical mycobacterial infections are more commonly
seen in children, often presenting as an inflammation of the lymph nodes. Rarely, M. bovis (bovine tuberculosis)
can be transmitted from infected animals (cattle, deer, possums and ferrets) to humans via handling or ingestion of contaminated
animal products, including raw milk, or by airborne droplet spread to people who work closely with animals.19
Symptoms of tuberculosis are dependent on the organ system involved, e.g. pulmonary, intestinal, bone, lymphatic system.
Pulmonary symptoms are most common, including dry cough which becomes productive, haemoptysis, pleuritic chest pain and
breathlessness, along with anorexia, fatigue, fever and night sweats.19
Patients with suspected tuberculosis should be discussed with an Infectious Diseases Specialist. Chest x-ray and sputum
culture are usually the initial tests. Further testing, e.g. QuantiFERON Gold assay, may also be required. Tuberculosis
is a notifiable disease so all suspected or confirmed cases must be notified to the local Medical Officer of Health.
Combination antibiotic treatment is required for up to one year, or longer in some cases.19 Tuberculosis
can remain latent for many years, and in some cases reactivation may occur years after the original exposure.19 People
with active pulmonary tuberculosis are infective to others for several months to years.19
 For further information see: “The
guidelines for tuberculosis control in New Zealand”, available from: www.health.govt.nz
For further information see: “The
guidelines for tuberculosis control in New Zealand”, available from: www.health.govt.nz

Bovine tuberculosis in New Zealand livestock
It is thought that bovine tuberculosis was first established in New Zealand in the 1800s when cattle and deer were
introduced. Control measures were implemented in the mid 1900s and by the 1970s all cattle herds were undergoing regular
testing for tuberculosis and post-mortem inspection for disease. Bovine tuberculosis was eradicated in several regions,
but there was unexplained disease in some areas, such as the West Coast of the South Island. It was found that livestock
were being infected via the Australian brush-tail possum, which was introduced into New Zealand in the 1870s. Possum
control measures were implemented in areas with persistent tuberculosis, which resulted in significant declines in livestock
infections. When possum control measures were later relaxed in the 1980s, bovine tuberculosis returned, peaking in the
mid-1990s at rates much higher than in other developed countries. In the past decade, renewed efforts to control bovine
tuberculosis and cooperation between herd owners have resulted in levels which are at an all-time low. It is hoped that
in the near future, New Zealand cattle herds will become “TB-free”. There have been no reported cases in New Zealand
in recent years of transmission of bovine tuberculosis from cattle to humans.
 For further information see: www.tbfree.org.nz
For further information see: www.tbfree.org.nz
Orf
Orf, also referred to as contagious ecthyma, contagious pustular dermatitis or scabby mouth, is a virus that commonly
affects sheep (usually lambs) and goats, that can be transferred to humans.20 It is caused by the parapoxvirus
orf virus.20 Other livestock, such as deer and cattle, are affected by similar poxviruses (see: “Milker’s
nodules”). Although orf can be a life-threatening disease in sheep and goats, it is a relatively mild and self-limiting
condition in humans.
Orf is most frequently seen in farmers, shearers, meat processors, veterinarians and people bottle-feeding lambs.21 Orf
is characterised by the development of a 2 – 3 cm tender, flat-topped, red-to-blue papule or pustule on the dorsum of
the index finger or hand (less commonly on the forearm or face), approximately one week after contact with an infected
animal (Figures 1 and 2).20, 21 The lesion will eventually crust over and resolve within
two months. Usually only one lesion develops, but in some cases there may be multiple lesions.21 Lymphadenopathy
may be present, along with red streaks marking the lymph channels.21 In some cases, patients may develop
erythema multiforme, which is a secondary rash on distal limbs, characterised by target lesions with central blistering.
The rash may persist for two to three weeks. Orf lesions may be more progressive and destructive in patients who are
immunocompromised.
Orf can be diagnosed based on the appearance of the lesion and a history of contact with animals; laboratory investigation
is not usually required. Standard microbiology culture will be negative. Skin biopsy typically shows ballooning of keratinocytes,
necrosis and inclusion bodies.
No specific treatment is indicated, unless secondary bacterial infection is present; staphylococcal infection is most
likely, which would be treated with flucloxacillin or cephalexin (see New
Zealand Formulary or bpacnz antibiotic guide for
further details). Lesions can be covered to prevent cross-contamination. Patients with large lesions may require shave
excision, and should be referred to a Dermatologist.21 There is some evidence that imiquimod cream is effective
in treating orf,22 however, this is an off-label use of this medicine and would not meet Special Authority
criteria for subsidy.
 For further information on orf and other parapox viruses, see Dermnet: www.dermnetnz.org
For further information on orf and other parapox viruses, see Dermnet: www.dermnetnz.org
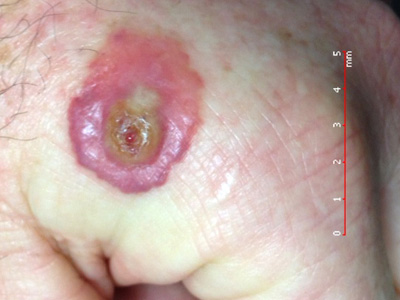
Figure 1: Typical orf lesion. Image provided by DermNet NZ (Courtesy of Dr
Bert Rauber) |
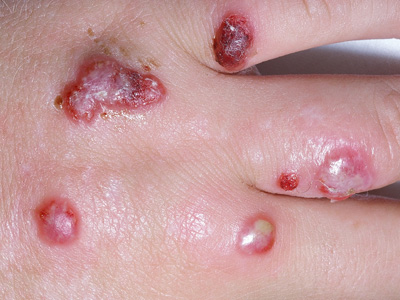
Figure 2: Multiple orf lesions. Image provided by DermNet NZ |
Milker’s nodules
Milker’s nodules are caused by a parapox virus that affects cattle. Infection is carried on the teats or in the mouth
of cows (“ring sores”) and can be passed to humans while milking or examining the animal.23 It is sometimes
referred to as “cowpock” and is often confused with cowpox, which is a viral skin infection caused by the vaccina-type
cowpox virus (part of the family of viruses that also includes smallpox).24 Cowpox is extremely rare and
unlikely to be seen in New Zealand.
Milker’s nodules develop 5 – 14 days after exposure to the virus. They begin as small, red, raised, flat-topped lesions,
and over the course of approximately one week, they become red-blue, firm, tender vesicles or nodules, that may develop
a greyish skin and small crust. The nodules usually appear on the hands, and less commonly on the face. There may be
one or two nodules, or several.23 As with orf, secondary bacterial infection and erythema multiforme may
occur in some cases.
Laboratory investigation is usually not required as milker’s nodules can be diagnosed based on the appearance of the
lesions and a history of contact with cattle. However, if there is any doubt about the diagnosis, a skin biopsy can be
performed.23
Management is the same as for orf. Nodules should be covered to prevent contamination, and patients advised to wear
gloves if milking. Antibiotic treatment may be required if secondary bacterial infection is present.23
 See: www.dermnetnz.org/viral/milkers-nodules.html for
images of milker's nodules
See: www.dermnetnz.org/viral/milkers-nodules.html for
images of milker's nodules
Dermatophyte infections: ringworm
A dermatophyte infection is a skin, nail or hair infection caused by fungi which use keratin for growth. Infections
may be acquired from a human (anthropophilic), animal (zoophilic) or soil (geophilic) source. Tinea corporis, known as
ringworm, is an example of a dermatophyte infection. The anthropophilic dermatophyte Trichophyton rubrum is
the most common cause of tinea corporis in New Zealand, and originates from infection in the feet (tinea pedis) or nails
(tinea unquium). Tinea corporis caused by T. rubrum most often affects people with lowered immunity, e.g. people
with diabetes or people treated with oral or topical corticosteroids. It is characterised by annular plaques which expand
slowly.
Microsporum canis (from cats and dogs) and T.verrocosum (from cattle) are the most commonly implicated zoophilic
dermatophyte infections responsible for tinea corporis.25 Patients with zoophilic (or geophilic) ringworm
usually present with single or multiple itchy, inflamed, skin lesions that form irregular expanding rings with a raised,
distinct border (Figure 3). There are often scattered follicular pustules and loss of hair within
affected areas. The lesions are usually located in exposed areas. Dermatophyte infections rarely occur on or near mucous
membranes, helping to differentiate them from candidal infections.26 Adults and children in rural areas may
present with kerion (fungal abscess – Figure 4).
Diagnosis of tinea corporis can be made by clinical appearance, but should be confirmed by laboratory analysis of skin
scrapings and extracted hair shafts. Patients should not use topical anti-fungal medicines for three days prior to a
sample being taken as this can prevent identification of the dermatophyte.
Patients with tinea corporis affecting a small area of skin can be treated with topical antifungals (e.g. miconazole
or clotrimazole cream). If topical treatment fails, the rash is extensive, there is follicular involvement or the patient
has kerion, oral antifungals are appropriate, e.g. terbinafine 250 mg, once daily, for four weeks – sometimes longer.
 For further information on collecting skin scrapings, see: “Collecting
specimens for the investigation of fungal infections”, Best Tests (Mar, 2011)
For further information on collecting skin scrapings, see: “Collecting
specimens for the investigation of fungal infections”, Best Tests (Mar, 2011)
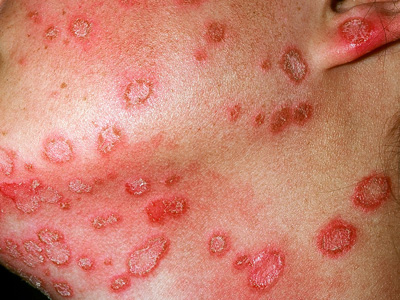
Figure 3: Zoophilic tinea corporis (M. canis) Image provided by DermNet
NZ |
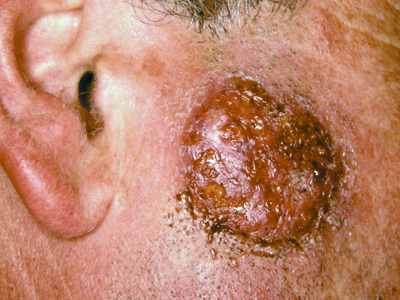
Figure 4: Kerion (T.verrocosum – cattle ringworm) Image provided by
DermNet NZ |
Erysipeloid infection
Erysipeloid is an infection caused by Erysipelothrix rhusiopathiae. It is transferred to humans via contact
with raw meat, poultry, fish and shellfish, when bacteria enter the skin through an open wound. Farmers, meat processors
and veterinarians are most at risk of infection.27
Patients with erysipeloid can be affected in three ways: most often they will present with localised skin lesions,
in very rare cases a diffuse cutaneous reaction occurs with multiple lesions across the body, and also rarely, a systemic
infection affecting multiple organs can occur. Localised lesions are red-purple, with a smooth, shiny surface. The lesions
slowly expand over several days, and develop a sharp or curved border, with very small blisters.27 The lesions
may feel warm, and pain, tenderness and a burning sensation may be reported.27 Most lesions occur on the
hands or fingers, but can form on any skin area exposed to the infected meat or animal.27
Laboratory investigation is not required; diagnosis is based on clinical examination. Lesions will resolve spontaneously
within two to four weeks.27 Antibiotic treatment can be considered to shorten the healing time. Oral flucloxacillin
is an appropriate treatment; erythromycin or doxycycline are alternatives.27
 Search: www.google.com/images for
images of Erysipeloid
Search: www.google.com/images for
images of Erysipeloid
Foreign body granulomas: wool handlers
A foreign body granuloma is a non-immunological reaction to an exogenous material (e.g. wood or metal fragment, fibres)
that has penetrated the skin. The foreign body is encapsulated within granulation tissue (which contains a proliferation
of inflammatory and giant cells) and can mimic a soft tissue tumour. In some cases, a sinus is formed, which can result
in infection.
Foreign body granulomas have been reported in people who handle sheep, e.g. wool handlers, shearers, pressers and rousies,
although there is little published literature on this. When the wool is handled, wool fibres (especially when wet) may
penetrate areas of exposed skin, e.g. the limbs and neck. This is also reported to occur in the breast and nipple area,
when fibres penetrate through clothing. The resulting painful, swollen lesion is colloquially referred to as a “grease
ball”. This condition is similar to trichogranulomas that affect hairdressers or dog groomers, when hair penetrates the
skin, usually between the fingers, and there is a foreign body reaction to the presence of keratin in the dermis.
A foreign body granuloma can be diagnosed with histopathology (fine needle aspiration or excision biopsy), which will
show characteristic cell formation. Foreign bodies can sometimes be detected on ultrasound, but this is unlikely to reveal
a wool fibre. Patients with infected lesions may require local incision and drainage, and antibiotics. Historically,
topical application of methylated spirits has been used as a treatment for “grease balls”. Protective clothing and gloves,
and the use of a barrier (moisturising) cream on exposed skin can help to prevent foreign body granulomas from occurring.
Infections acquired via contact with plants or soil
There are many infectious pathogens which pose a risk to people working in outdoor occupations. For example, bacterial
or fungal skin infections can occur in crop and field workers, and there is a risk of tetanus being transferred to a
wound from soil. Some less common skin and soft-tissue infections are contracted via water-borne microbes through minor
abrasions, e.g. Aeromonas hydrophila, a rare cause of cellulitis and abscess, and Mycobacterium marinum,
a cause of chronic granulomatous plaques.
 For further information on Aeromonas skin infection see:
www.dermnetnz.org/bacterial/aeromonas.html
For further information on Aeromonas skin infection see:
www.dermnetnz.org/bacterial/aeromonas.html
Paronychia
Horticultural workers are at risk of skin infections due to repeated minor trauma, e.g. from thorns and vines. Paronychia
is inflammation of the nail folds, caused by bacterial, viral or yeast infection of the fingers or, less commonly, the
toes.28 It occurs when there is penetration between the proximal nail fold and the nail plate, allowing microbial
entry. Disruption of the nail seal can also occur due to a contact irritant or excessive moisture.28
Paronychia can be acute or chronic. Acute paronychia is caused by bacterial infection, most commonly Staphylococcus
aureus, and sometimes Streptococci and Pseudomonas organisms,28 or by herpes simplex
virus. Chronic paronychia is when symptoms have been present for more than six weeks, and is usually due to a fungal
infection, e.g. Candida albicans. It is more likely in people who have repeated exposure to water containing
chemical irritants or exposure to moist environments.28 Chronic paronychia may also arise as a complication
of hand dermatitis.
Patients with acute paronychia (Figure 5) present with localised pain, tenderness and swelling
of the perionychium (epidermis bordering the nails). Discharge may be present if an abscess has formed and infection
may extend into the nail bed. The nail may be discoloured or distorted.28 Laboratory investigation is not
required unless the infection is severe. If there are signs of significant bacterial infection, oral antibiotic treatment
is recommended; flucloxacillin is an appropriate choice. Incision and drainage is recommended if there is an abscess.28
In chronic paronychia (Figure 6), several nails and perionychium appear swollen and tender, with
“boggy” nail folds. There is thickening, transverse ridging and discolouration of the nail plate, and separation of the
nail from the cuticle and nail folds.28 Microbiological analysis of nail scrapings can be considered to identify
the causative agent. Treatment with a combination of topical corticosteroids and a topical antifungal (when yeast infection
is present) is usually successful. If symptoms do not resolve, an oral azole antifungal or antibiotic, depending on the
microbes present, can be considered. If medical treatment is unsuccessful and the case is severe, surgical intervention
may be considered; this may involve removal of the nail.28
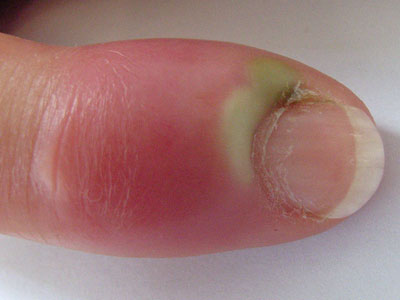
Figure 5: Acute paronychia. Image provided by DermNet NZ |
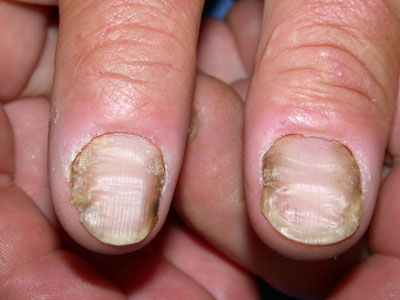
Figure 6: Chronic paronychia. Image provided by DermNet NZ |
Tetanus
Clostridium tetani, the causative organism of tetanus, is present in soil, dust and animal faeces. People
are at risk of tetanus if infected soil or other matter enters a wound. Once in an anaerobic environment in the wound, C.
tetani multiplies and releases a toxin which causes the characteristic symptoms of tetanus: muscular rigidity and
contraction spasms. Symptoms develop 3 – 21 days after exposure (ten days on average).29 Initial symptoms
include weakness, stiffness or cramps and patients may report difficulty chewing or swallowing food. Muscle spasms usually
begin one to four days later. The mortality rate for people with tetanus is approximately 10%, but is higher in older
people.29
Tetanus is rare in New Zealand due to an effective immunisation programme which was introduced for infants in 1960.29 Prior
to this, only people in the armed forces were likely to have received a primary series of tetanus vaccinations. Most
cases of tetanus occur in older people (particularly older women) as they are less likely to have been immunised or to
have received booster vaccinations. Between 2000 and 2010, there were 34 people in New Zealand hospitalised with tetanus;
23 of these people were aged over 60 years.29
If a patient presents with a tetanus-prone wound, it should be cleaned and dressed, and they should receive a tetanus
booster immunisation if they have not had one within the last five to ten years (Table 1). Td (ADT
Booster) or Tdap (Boostrix) can be used. Patients with no history of previous tetanus immunisation and a tetanus-prone
(“dirty”) wound should receive a primary course of tetanus vaccination (three doses) and should also receive tetanus
immunoglobulin (TIG). The recommended dose is 250 IU, IM (one ampoule), but this should be increased to 500 IU if the
wound occurred more than 24 hours previously or if there is a risk of heavy contamination.29
Patients with features suggestive of tetanus should be referred to hospital for further assessment and management.
Table 1: Guide to tetanus prophylaxis in wound management (adapted from Immunisation Handbook,
2011)29
| Vaccine history |
Time since last dose |
Type of wound |
Tetanus vaccination required? |
Tetanus immunoglobulin (TIG) required? |
| ≥ 3 doses |
< 5 years |
Tetanus-prone |
No |
No |
| ≥ 3 doses |
5 – 10 years |
Clean/minor |
No |
No |
| ≥ 3 doses |
5 – 10 years |
Tetanus-prone |
Booster dose |
No |
| ≥ 3 doses |
> 10 years |
Tetanus prone |
Booster dose |
No |
| < 3 doses |
|
Clean/minor |
Complete course of three doses |
No |
| < 3 doses |
|
Tetanus-prone |
Complete course of three doses |
Yes |

Assess tetanus status at age 45 and 65 years
The tetanus immunisation status of adults should be reviewed at age 45 and 65 years. If it has been more than ten
years since receiving a tetanus vaccination, patients should be offered a booster vaccination: Td (ADT Booster) or Tdap
(Boostrix). If they do not have a reliable history of tetanus vaccination a primary course should be given, which is
three doses of Td or Tdap, at least four weeks apart. A booster dose is then recommended in ten years.