In this article 
View
/ Download pdf version of this article
The incidence of syphilis in New Zealand has increased dramatically over the last decade. As
syphilis is highly infectious in the early stages, prompt identification is important. However, the perceived rarity of
syphilis, combined with often non-specific symptoms, mean this can be difficult. Recognising the signs and risk factors
and incorporating syphilis testing into sexual health checks, is essential for controlling the growing number of new syphilis
infections.
A recent increase in new syphilis infections
Syphilis is a sexually transmitted infection caused by the spirochete (spiral shaped) bacterium Treponema pallidum.
It is unknown where and when syphilis first emerged, but one theory is that explorers carried syphilis from the “New
World” back to Europe with them in the 1400s, where it spread so quickly it became known as the Great Pox. Many
famous historical figures are thought to have had syphilis, including Vincent van Gogh, Adolf Hitler and Leo Tolstoy,
and prostitutes infected with syphilis are most likely the origin of the “femme fatale” character in literature.
It was not until the discovery of penicillin in the 1940s that the prevalence of syphilis began to decline.1
In New Zealand, less than 20 cases of syphilis were being reported each year by the early 2000's.2 However,
the incidence of syphilis is now beginning to increase again, with the most recent data showing that 119 cases were reported
by sexual health clinics in New Zealand in 2010.3,4 While in absolute terms these numbers are low, they represent
a 600% increase in prevalence in less than a decade. Similar increases have been occurring worldwide and have been attributed
to factors such as decreases in the resources allocated to the diagnosis and control of syphilis, increased international
travel and increases in the number of men who have sex with men.1
Although syphilis is still a relatively rare condition, it should be considered as part of a sexual health check. Syphilis
in New Zealand is most commonly seen in men who have sex with men (in particular, HIV positive men), in heterosexual people
who have sex while overseas, particularly in South East Asia and Africa and in immigrants, particularly those from the
Asia-Pacific region.
The symptoms of syphilis
Syphilis is termed the “great imitator”, as symptoms are often non-specific or mimic other infectious or
immune mediated conditions, e.g, the rash seen in secondary syphilis may resemble pityriasis rosea. Approximately 50%
of people with syphilis are asymptomatic.
The progression of syphilis is divided into three stages; primary, secondary and tertiary. An asymptomatic latent period,
which may last more than a decade, separates the secondary and tertiary stages. The time between infection and development
of initial symptoms is on average 21 days, but ranges from 10 - 90 days.5 People with syphilis are highly infectious
during the primary and secondary stages, with infectivity declining in the latent and tertiary stages. Syphilis spirochetes
are able to pass through intact mucous membranes and compromised skin, so are transmissible via kissing and vaginal, oral
and anal sex.1 Consistent condom use reduces, but does not eliminate, the risk of syphilis infection.6 It
is estimated that the rate of transmission between people with primary or secondary syphilis and their sexual partners
is 30%.1
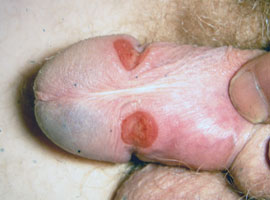 Figure 1: Penile chancre in primary
syphilis (Supplied by Dermnet NZ)
Figure 1: Penile chancre in primary
syphilis (Supplied by Dermnet NZ) |
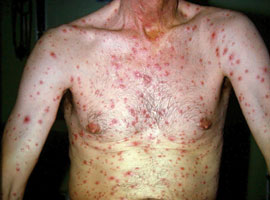 Figure 2: Disseminated rash in secondary
syphilis Pox (Supplied by Dermnet NZ /Dr John Adams)
Figure 2: Disseminated rash in secondary
syphilis Pox (Supplied by Dermnet NZ /Dr John Adams) |
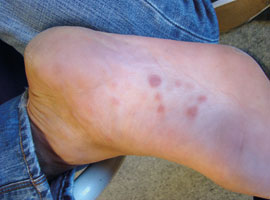 Figure 3: Characteristic rash on
the foot in secondary syphilis. (Supplied by Dr Edward Coughlan)
Figure 3: Characteristic rash on
the foot in secondary syphilis. (Supplied by Dr Edward Coughlan) |
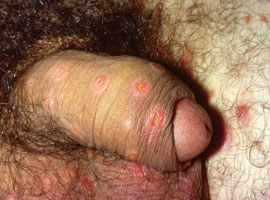 Figure 4: Condylomata lata in secondary
syphilis (Supplied by Dermnet NZ/Dr John Adams)
Figure 4: Condylomata lata in secondary
syphilis (Supplied by Dermnet NZ/Dr John Adams) |
Primary syphilis
The initial stage of syphilis is typically marked by the appearance of a single chancre (ulcer-like lesion), although
multiple lesions may be present (Figure 1).7 The chancre is firm, painless and can vary in size up to approximately
3 cm. It generally appears at the site of disease transmission, and therefore may not be noticed by the patient if it
is inside the vagina, anus or oral cavity. Non-tender lymphadenopathy may develop near the site of the chancre.
The chancre typically resolves within four to eight weeks and does not require localised treatment, although antibiotic
treatment to prevent syphilis infection from progressing is necessary (below).7
Secondary syphilis
Secondary syphilis develops three weeks to three months after the appearance of primary syphilis, if left untreated.7 Secondary
syphilis is characterised by skin rashes and mucous membrane lesions. The typical rash is a widespread, symmetrical eruption
of slightly scaly, reddish brown plaques (Figure 2) that also occurs on the palms of the hands and the soles of the feet
(Figures 3). However, rashes may be non-specific, appear on other parts of the body or resemble rashes caused by other
conditions, such as pityriasis rosea. Rashes may be so faint that they are not noticed.
Condylomata lata may also be present. These are moist, grey, pink or white, raised, wart-like lesions or plaques which
are highly infectious areas of concentrated spirochete particulates. They can occur on the penis (Figure 4), vulva, rectum,
mouth, throat, larynx, inner thighs, armpits and under breasts.7
Other symptoms of secondary syphilis are non-specific and include flu-like symptoms, such as lymphadenopathy, tiredness,
headache, sore throat, fever and weight loss.7,8
The rashes and lesions associated with secondary syphilis typically resolve within two to six weeks. Antibiotic treatment
reduces the duration of symptoms and prevents progression to tertiary disease.8
Tertiary syphilis
Approximately one-third of untreated people will develop tertiary syphilis. This stage occurs after a latent period,
when infection is identifiable on serological testing but the patient does not have symptoms or signs. The tertiary stage
usually appears within three to ten years after syphilis was first acquired, although it can appear up to 40 years later.1
Signs, symptoms and long term sequelae of tertiary syphilis include:1,7
- Neurological infection (neurosyphilis): numbness in arms, legs and face, paralysis, gradual blindness, changes in
mental state and eventually dementia
- Cardiovascular disease: classically chronic inflammation of the aorta resulting in aneurysm formation, aortic valve
incompetence and, in the longer term, congestive heart failure
- Granulomatous lesions (gummas): painless rubbery nodules mostly seen on the skin, mouth and throat that may ulcerate,
or form as lesions in the long bones, which typically cause bone pain at night
The Oslo study estimated the probability of dying as a result of untreated syphilis to be 17% in males and 8% in females
after 40 years infection.9 The infamous Tuskagee study, which exploited a vulnerable patient population and
withheld treatment after it became available, found that after 20 years of follow up, cardiovascular disease or neurosyphilis
was the primary cause of death in 30% of African American males with syphilis.10
Determining the risk of exposure to syphilis
As part of a sexual health check, ask about behaviours or factors that may increase a person's risk of exposure to syphilis.
People with an increased risk of syphilis include those who:
- Originate from a country where syphilis is common, e.g. Sub-Saharan Africa, Asia-Pacific (especially Fiji), South
America or Eastern Europe
- Have had sex with a person from a country where syphilis is prevalent
- Are male and have had sex with other males
- Are HIV positive or have had sex with someone who is HIV positive
- Have multiple sexual partners
- Have had sexual contact with a person diagnosed with syphilis
Patients at increased risk should be examined for signs and symptoms of syphilis. Syphilis serology (below)
should be requested, along with other STI investigations, including HIV.7 Ulcer-forming genital diseases, such
as syphilis, increase the likelihood of transmission of HIV and other STIs by five to ten times.5,11
Specific signs which indicate an increased likelihood of syphilis include:
- Chancre (microscopy required, see below)
- Condyloma lata
- Rash on the palms of hands and on the soles of feet (strongly suggestive of secondary syphilis)
- Rash on the torso or lymphadenopathy not attributable to another condition
Yaws in the Asia Pacific region
Treponemal bacteria are responsible for a number of diseases other than syphilis, such as yaws, bejel and pinta. All
have similar symptoms and outcomes to syphilis, but are not sexually transmitted.12 These diseases are endemic
to many tropical regions, but World Health Organisation eradication efforts have led to a greatly reduced number of people
with new infections.12
Yaws is likely to be the most common non-syphilitic treponemal infection to be seen in immigrants to New Zealand, particularly
those from the Pacific and Asia. While the likelihood of seeing a patient with yaws is very low, many may have been exposed
to this infection during childhood. The antibodies produced by the immune system in response to yaws remain present for
life.12 As such, people with a previous yaws infection will always return a reactive result on specific serology
testing (below). This should be taken into consideration if investigating for syphilis in a patient who has immigrated
from a country or region with an increased prevalence of any tropical treponemal disease.
Laboratory testing for suspected syphilis
Syphilis serology should be requested in patients at increased risk or with clinical features suggestive of syphilis.
Patients with a suspected chancre should also be referred to a sexual health clinic or laboratory for assessment and microbiological
examination of the lesion.
N.B. Syphilis serology is also included in the first antenatal screen in women who are pregnant and is required as part
of an immigration medical examination.
Serological testing
There are two types of syphilis serology test - non-specific (non-treponemal) serology and specific (treponemal) serology.
Non-specific tests detect antibodies that bind to antigens that are, or are similar to, those expressed by Treponema pallidum
or expressed on host tissues during infection. These tests, such as the Rapid Plasma Reagin (RPR) and Venereal Disease
Research Laboratory (VDRL) test, were traditionally used as screening tests for syphilis, and to measure disease activity
and response to treatment.13 They are inexpensive to perform (compared to specific tests) but have a high false-positive
rate, particularly in women who are pregnant, in people with cancers, autoimmune disorders, co-morbid viral infections,
in older people and in people who use illicit drugs.7,14
Specific tests detect antibodies that bind to proteins derived from Treponema pallidum. These tests, such as the Treponemal
pallidum Particle Agglutination (TTPA), Treponema pallidum Haemagglutination (TPHA) and Fluorescent Treponemal Antibody
(FTA) test, have commonly been used to confirm the diagnosis of syphilis.13 They are more expensive than non-specific
tests, but have a low false-positive rate. More recently, the Enzyme immunoassay (EIA) and derivative immunoassays, such
as the Chemiluminescent Microparticle Immunoassay (CMIA), that use specific Treponema pallidum antigens, have been developed.
These tests are less expensive and have altered the way serology is used for testing for syphilis.
The approach generally used by laboratories in New Zealand is to perform an initial test with EIA. If this is positive,
the diagnosis is confirmed using TPPA. Disease activity is then determined using RPR.14,15 Depending on the
patient-management system in use and the methodology of the local laboratory, clinicians either select “syphilis
serology” on the laboratory request form or request the individual tests.
Antenatal screening and congenital syphilis
Congenital syphilis occurs when infection is passed vertically from mother to infant in-utero. The risk of transfer
in-utero is approximately 75 - 95% in a mother with primary syphilis. The risk of a mother passing infection to a foetus
remains for up to seven years post infection, if untreated.8 The mother does not need to be symptomatic to
pass on the infection.
Serology testing for syphilis is included in the first antenatal screen. Testing should be repeated at 28 weeks and
prior to delivery in women with a high risk of syphilis infection, such as recent immigrants from high-risk countries.5
Interpreting syphilis serology
Syphilis serology results should be interpreted within the overall clinical picture, i.e. clinical examination, patient
history and risk profile. Table 1 may be useful in aiding interpretation.
Table 1: Interpreting syphilis serology
| EIA |
TPPA |
RPR |
Interpretation |
| Non-Reactive |
Not tested |
Not tested |
No evidence of syphilis, or too early, retest in one month if strong suspicion based on clinical
evidence
|
| Reactive |
Non-Reactive |
Non-Reactive |
Possible early primary, latent or false-positive, retest in one month
|
| Reactive |
Non-Reactive |
Reactive |
Probable early primary, false positive possible but unlikely, retest in two weeks
|
| Reactive |
Reactive |
Non-Reactive |
Evidence of past infection or possible latent infection, history will help to differentiate |
| Reactive |
Reactive |
Reactive |
Current syphilis |
EIA, TPPA and RPR results are expressed as “reactive” or “non-reactive”. RPR results also include
a titre, with higher titres indicating greater disease activity. N.B. People who have had a past treponemal infection,
including non-venereal infections such as yaws, will remain reactive on specific treponemal tests for their lifetime.7,14
Discussion with a sexual health or infectious disease physician is recommended if results are contradictory or difficult
to interpret.
Referral for microscopy is required for patients with a chancre
If a patient presents with a suspected chancre, they should be referred to a sexual health clinic or laboratory for
assessment and examination of the exudate from the lesion. Dark field microscopy is used to detect bacterium particulates
(spirochetes).7
Exudate from oral and anal chancres cannot be reliably examined with standard dark-field microscopy due to the likelihood
of contamination. Direct fluorescent antibody (DFA) stains for microscopy can be used,16 however, the availability
of this method is variable.
Topical treatment should not be applied to the chancre and systemic antibiotics should not be prescribed until the sample
has been taken as this may alter the results.16
Syphilis serology should also be requested.
Management of syphilis: refer for treatment
Patients who have tested positive for syphilis should be referred urgently to a sexual health or infectious disease
physician for treatment.14
Benzathine benzylpenicillin injection is the first-line treatment for syphilis at all stages. Tetracyclines, some macrolides
and cephalosporins can be used as an alternative if the patient is allergic to penicillin. Penicillin is the only recommended
treatment in women who are pregnant. Skin testing for penicillin allergy followed by de-sensitisation is recommended for
pregnant women with a history of penicillin allergy.7
Effective treatment usually results in a decline in RPR titres, although a return to pre-infection levels may take many
years.
Follow-up and partner notification
Sexual partner notification is necessary for all people diagnosed with syphilis. This is usually carried out by the
sexual health clinic.
If the patient has primary syphilis, all sexual partners within the last three months should be contacted, offered testing
and begun on empiric treatment, regardless of serology results.14
If the patient has secondary syphilis, all sexual partners within the last six months should be contacted and offered
testing. Empiric treatment should be commenced if sexual contact has been within the last three months, regardless of
serology results.
If the patient has early-latent syphilis or syphilis of unknown duration with an RPR titre of 1:32 or more, all sexual
partners within the last 12 months should be contacted and offered testing. Empirical treatment should be commenced if
sexual contact has been within the last three months regardless of serology results.14
In most cases, patients with late-latent or tertiary syphilis are unable to transmit syphilis infection. Current partners
and children of women with late-latent or tertiary syphilis should be tested.
Syphilis is not a Notifiable Disease, but surveillance of cases is performed by Environmental Science and Research Ltd
(ESR) through voluntary reporting by laboratories and sexual health clinics.
ACKNOWLEDGEMENT: Thank you to Dr Edward Coughlan, Clinical
Director, Christchurch Sexual Health, Canterbury DHB, Associate Professor Mark Thomas, Infectious Diseases
Specialist, Department of Molecular Medicine and Pathology, University of Auckland, Dr Sunita Azariah Sexual
Health Physician, Auckland Sexual Health Service and Dr John Bannister, Registrar, Auckland Sexual Health
Service, for expert guidance in developing this article.
References
- Walker G. Antibiotics for syphilis diagnosed during pregnancy. Cochrane Database of Syst Rev 2010;(3):CD001143.
- The Environmental Institute of Science and Research (ESR). Sexually transmitted infections in New Zealand: 2001.
Ministry of Health; 2002. Available from: www.surv.esr.cri.nz (Accessed Jun, 2012).
- The Environmental Institute of Science and Research (ESR). Sexually transmitted infections in New Zealand: 2003.
Ministry of Health; 2004. Available from: www.surv.esr.cri.nz (Accessed Jun, 2012).
- The Environmental Institute of Science and Research (ESR). Sexually transmitted infections in New Zealand: 2010.
Ministry of Health; 2011. Available from: www.surv.esr.cri.nz (Accessed Jun, 2012).
- Kollmann TR, Dobson S. Chapter 16 - Syphilis. Infectious Diseases of the Fetus and Newborn (7th Ed). Philadelphia:
W.B. Saunders; 2011. p.524-63.
- Koss C, Dunne E, Warner L. A systematic review of epidemiologic studies assessing condom use and risk of syphilis.
Sex Transm Dis 2009;36(7):401-5.
- Ngan V. Syphilis. DermNet NZ. 2011. Available from: www.dermnetnz.org/bacterial/syphilis (Accessed Jun, 2012).
- Longo D, Fauci A, Kasper D, et al. Harrison's principles of internal medicine. 18th ed. New York: Mc Graw Hill Medical;
2012. p. 1384-8.
- Clark EG, Danbolt N. The Oslo study of the natural history of untreated syphilis: An epidemiologic investigation
based on a restudy of the Boeck-Bruusgaard material a review and appraisal. J Chronic Dis. 1955;2(3):311-44.
- Olansky S. Untreated syphilis in the male Negro: Twenty years of clincial observation of untreated syphilitic and
presumably non-syphilitics groups. J Chronic Dis. 4(177):1956.
- Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Euro J Int Med 2009;20(1):9-13.
- Lal S, Dhariwal AC. Yaws, Pinta, and Endemic Syphilis. Int Encyclo Pub Health. Oxford: Academic Press; 2008. p. 651-7.
- Hoover K, Radolf J. Serodiagnosis of syphilis in the recombinant era: Reversal of fortune. J Infect Dis 2011;204(9):1295-6.
- The New Zealand Sexual Health Society (NZSHS). Syphilis. NZSHS: Auckland; 2009. Available from: www.nzshs.org/treatment_guidelines/Syphilis_2009.pdf
(Accessed Jun, 2012).
- Editor: Kyle C. A handbook for the interpretation of laboratory tests. 4th ed. Diagnostic Medlab; 2008.
- Brown D, Frank J. Diagnosis and management of syphilis. Am Fam Physician 2003;68(2):283-90.