Serum protein bands (monoclonal gammopathy) will sometimes be found following serum protein
electrophoresis in patients presenting with classic signs or symptoms of multiple myeloma, e.g. bone pain. In other cases,
patients may have non-specific symptoms, meaning the discovery is more unexpected. Irrespective of how the band is discovered,
there are additional tests that can be performed to provide more information to help with the diagnosis and to guide management.
These tests include; immunofixation, Bence-Jones protein and serum free light chains.
While we may recognise the names of some of the tests, it can be confusing to know the situations in which they should
be requested, and even what the results mean. This article provides an overview of the clinical situations in which a
patient may have a monoclonal gammopathy in their serum, what these results mean, and the role of other related laboratory
tests.
In a general practice setting, test results showing increased protein in serum or urine are often incidental findings
when investigations have been requested for other reasons. Patients with clinical conditions such as multiple myeloma,
that increase the levels of protein in serum or urine, may present with a wide range of symptoms or may be asymptomatic.
In these patients serum protein electrophoresis may have been requested as part of the initial work-up.
Levels of protein in the serum change in a predictable way in response to many clinical conditions, such as inflammation,
trauma (including burns and chemical injury), malignancy, infarction and necrosis.1 Serum protein electrophoresis
is a laboratory test used to help identify patients with multiple myeloma and other serum protein disorders, by separating
serum proteins into their various components. Indications for serum protein electrophoresis are numerous and are based
on clinical, laboratory and occasionally radiological findings (see “Indications for requesting serum protein electrophoresis”).
Indications for requesting serum protein electrophoresis1,2,3
Indications based on clinical findings:
- Suspected multiple myeloma, Waldenström’s macroglobulinemia, primary amyloidosis or other related disorders
- Unexplained bone pain or fracture
- Recurrent infections
- Unexplained peripheral neuropathy (not able to be attributed to another condition, e.g. type 2 diabetes, chemotherapy)
Indications based on laboratory findings:
- High (or low) total serum globulin or immunoglobulin
- Extremely high percentage of lymphocytes
- Incidental finding of an increased total protein level
- Unexplained anaemia (multiple myeloma is a recognised cause of non-iron deficiency anaemia) or other persisting cytopaenias
for which there is no other explanation
- Unexplained high ESR (>50) with a normal CRP
- Unexplained hypercalcaemia or renal impairment
- Red cell rouleaux formations noted on the peripheral blood smear
- Unexplained high urine protein with relatively low or normal urine albumin
- Presence of urine free light chains (Bence-Jones proteinuria)
Indications based on radiological findings:
- Lytic lesions in bone
- Unexplained osteopaenia (as not all patients with multiple myeloma will have osteolytic lesions)
Electrophoresis fractions
Serum protein electrophoresis separates serum proteins into the following fractions: albumin, alpha, beta and gamma
globulins. Approximately 60% of the total protein in the serum is albumin, while the remaining fractions are composed
mainly of globulins, predominantly immunoglobulins. The gamma fraction contains the largest portion of immunoglobulins,
hence an increase in gamma globulin is referred to as a gammopathy.
Gammopathies can be either polyclonal, characterised by a broad diffuse band in the electrophoresis pattern, or monoclonal
where the band is sharp and well defined. Making the distinction between a monoclonal and polyclonal gammopathy is important.
Monoclonal gammopathies are associated with excessive production of immunoglobulins from a single clone of cells that
is malignant or potentially malignant, whereas polyclonal gammopathies are characterised by a generalised increase in
immunoglobulins.1 A polyclonal gammopathy can be caused by various infections, haematologic diseases, liver
disease, some malignancies and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, temporal
arteritis and sarcoidosis.
This article focuses on monoclonal gammopathies, and provides guidance on the laboratory management of conditions associated
with these.
Monoclonal gammopathy
Monoclonal gammopathy is the name given to a “band” in serum protein electrophoresis, caused by the overproduction
of a population of plasma cells, which in turn produce a single immunoglobulin (the so-called “plasma cell dyscrasias”).
As there is a finite capacity in the bone marrow, an enlarging clone of plasma cells expands at the expense of other cells.
Levels of other normal immunoglobulins eventually fall, referred to as immune paresis. While this is most often associated
with multiple myeloma, it is quite frequently an unexpected discovery, and may be related to a number of conditions.
Conditions associated with monoclonal gammopathy
There are a number of conditions associated with monoclonal gammopathy. In the first instance, most GPs will think of
multiple myeloma, but this is less common, and most people will be found to have a monoclonal gammopathy of undetermined
significance (MGUS). Table 1 compares the incidence of these conditions.
| Table 1: Conditions with monoclonal gammopathies6,7,8 |
| Condition |
Clinical Effect |
Incidence |
| Monoclonal gammopathy of undetermined significance (MGUS) |
Asymptomatic with risk of progression |
1% >50 years, rising up to 8% >70 years |
| Multiple myeloma |
Severe |
40 per million, but increases to 300 per million for people >80 years |
| Waldenström’s macroglobulinaemia |
Moderate |
0.1 per million at age <45 years and 36.3 per million at age >75 years. |
| Amyloidosis |
Severe |
8 per million |
Monoclonal gammopathy of undetermined significance (MGUS)
MGUS is the most common monoclonal gammopathy. It describes the presence of a monoclonal protein without sufficient
clinical or laboratory evidence to diagnose one of the other associated conditions. It is the most frequent diagnosis
of a monoclonal gammopathy, and has an incidence approximately 60 times greater than multiple myeloma (1% in people aged
over 50 years, rising to up to 8% of people aged over 70 years).4,5
Approximately 1% of patients per year with MGUS will progress to multiple myeloma, therefore periodic (usually annual)
monitoring should be done (with serum protein electrophoresis, immunoglobulins and complete blood count). There is no
plateau time, beyond which development of a condition such as multiple myeloma will not occur. However, reactive bands,
which can occur as part of the immune response to an inflammatory stimulus, often reduce or disappear when followed.
Multiple myeloma
Multiple myeloma is uncommon and has an incidence of approximately 40 per million people. It is rare under the age 40
years, but its incidence rises to over 300 per million in people aged over 80 years. The median age at diagnosis is 69
years with a slight male predominance.3
Patients with multiple myeloma can be classified as having asymptomatic (formerly known as smouldering) or symptomatic
(active) disease.
Anaemia and bone marrow failure, infections, renal impairment, bone pain and pathological fractures are common clinical
features. The differential diagnosis of multiple myeloma is shown in Table 2.
| Table 2: Differential diagnosis of multiple myeloma (adapted from O’Connell, et
al 2005)1 |
| Disease |
Distinctive features |
| Multiple myeloma (active) |
Monoclonal gammopathy in serum or urine – plasma concentration >30 g/L (IgG or A) or lower
concentration of monoclonal IgD or light chain band
and
≥10% plasma cells in bone marrow
and
evidence of organ dysfunction involving one or more of: lytic bone lesions or osteoporosis, anaemia, hypercalcaemia
or renal disease |
| Asymptomatic myeloma (smouldering) |
Monoclonal gammopathy ≥30 g/L (IgG) and/or ≥10% plasma cells in bone marrow
but
no evidence of disease-specific end-organ damage – no lytic bone lesions, anaemia, hypercalcaemia or renal disease |
| Monoclonal gammopathy of undetermined significance |
Monoclonal gammopathy <30 g/L and <10% plasma cell in bone marrow
and
no evidence of disease-specific end-organ damage – no lytic lesions, anaemia, hypercalcaemia or renal disease |
| Waldenström’s macroglobulinaemia |
IgM monoclonal gammopathy, and ≥10% bone marrow infiltration with lymphoplasmacytic cells (with
characteristic immune phenotype)
Clinical features include hyperviscosity, anaemia, and enlargement of liver, spleen and lymph nodes |
Less common associations of monoclonal gammopathies
Lymphomas: monoclonal gammopathy is a common feature of primary lymphoproliferative conditions such
as chronic lymphocytic lymphoma.
Waldenström’s macroglobulinaemia: a type of small cell lymphoma associated with production
(often large amounts) of monoclonal IgM. The median age at presentation is 63 years, and over 60% of patients are male.
Clinical features include enlargement of liver and spleen and anaemia, due to increasing concentration of IgM, and hyperviscosity
syndrome.3
Amyloidosis: Primary amyloidosis is associated with a monoclonal gammopathy in 85% of cases and is
characterised by pathological deposits of monoclonal light-chain fragments in various tissues such as heart, liver, bone
marrow, lymph nodes and bowel.
Plasmacytoma: refers to a localised solid collection of plasma cells in the body outside the bone marrow.
Laboratory tests for monoclonal gammopathies
There are number of laboratory tests which are useful for determining the presence of a monoclonal gammopathy, and then
eventually characterising it.
Serum total protein and albumin
These are relatively crude tests, but will often be abnormal if a monoclonal gammopathy and/or immune paresis is present.
A large band may show as a high serum total protein with a raised calculated globulin result. If the total serum protein
is very high, e.g. >90 g/L, protein electrophoresis may be performed on a reflex basis by the laboratory.
Immunoglobulins
A person with a monoclonal gammopathy will have an increase in a particular class of immunoglobulin: IgG, IgA, IgM or
IgD (IgE monoclonal gammopathy is extremely rare). Quantitation of immunoglobulins (routinely IgG, IgA and IgM) is performed
if a new monoclonal gammopathy is detected. It is also usually performed when following a known monoclonal gammopathy,
to provide information about progression.
Serum protein electrophoresis
Serum protein electrophoresis is a means of separating serum proteins. A small amount of serum is placed on a specific
medium (such as agarose) and an electrical charge is applied. The proteins then migrate across the medium in a characteristic
manner, due to the net charge and size and shape of the protein.
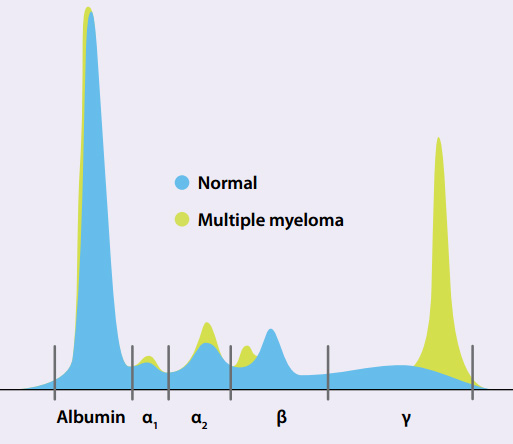
In routine serum protein electrophoresis, the protein will separate into five main components (Figure 1), identified
as albumin and the globulins (alpha1, alpha2, beta and gamma) . The gamma region contains the largest portion of globulins,
therefore monoclonal gammopathies are most frequently encountered in this portion of the electrophoresis.
Immunofixation
When serum protein electrophoresis identifies a mono-clonal gammopathy, the laboratory will automatically perform immunofixation
(i.e. reflex test) to further determine the exact type of monoclonal protein. The heavy chain of the immunoglobulin will
be identified as IgA, IgG or IgM (most commonly) or IgD (or IgE rarely). The light chains will be identified as kappa
or lambda (κ or λ). In a minority of cases only light chains (without heavy chains) are produced. Light chain-only
monoclonal gammopathy are often barely visible in serum but may show as large amounts of monoclonal light chains excreted
in the urine; hence the need to consider urine testing when clearly suspecting a monoclonal gammopathy.
Urine free light chain testing (Bence-Jones protein)
Even in disease-free people, light chains are produced in small excess over heavy chains, creating a surplus of (polyclonal)
free light chains. In a person with a monoclonal gammopathy this is often more marked, due to dysregulation of light versus
heavy chain production. All of the excess light chains also have an identical class and mobility. Excess free light chains
are frequently detectable in the urine by electrophoresis and immunofixation. The presence of monoclonal urine light chains,
often referred to as Bence-Jones protein, is found nearly exclusively in patients with lymphoproliferative processes such
as multiple myeloma.
Serum free light chains
Serum free light chain assays can detect normal levels of light chains in the blood, as well as elevated levels, even
when those levels are undetectable by serum protein electrophoresis and immunofixation. Both free κ and λ chains
are measured and the ratio is calculated. Excessive free κ or λ increases the likelihood a of monoclonal
plasma cell disorder.
Some evidence suggests that in patients with newly identified MGUS, an abnormal light chain ratio increases the likelihood
of progression independent of other factors such as band size and type. However, formal guidelines differ as to the use
of serum free light chains in this setting. Recent British Haematology Society guidelines do not recommend their use,
other than in patients who are otherwise at higher risk of progression (see “Monitoring monoclonal
gammopathy” below) and those where a malignant plasma cell disorder is otherwise clearly suspected.
Serum free light chains are useful in certain specific and uncommon settings, e.g. concern over a possible non-secretory
myeloma or amyloidosis, after specialist consultation. Light chains are sometimes used in specialist settings to monitor
the response of mutiple myeloma to treatment.
Serum free light chains are not recommended as a first line routine test for plasma cell disorders. There is also no
current evidence to support their use in long-term monitoring,9 except for monitoring response of mutiple myeloma
to treatment.
Clinical presentation of monoclonal gammopathy
Asymptomatic patients with chance findings
Approximately 30% of patients with monoclonal gammopathy are asymptomatic at diagnosis. In these cases the diagnosis
is made due to an unusual result being noticed by either the laboratory or the GP. Some of the more suspicious laboratory
findings include:
- Unexplained raised ESR (a monoclonal gammopathy does not increase CRP)
- Increased rouleaux formation on the blood film
- Increased serum total protein and/or calculated globulin (total protein minus albumin)
- Elevated immunoglobulin result
N.B. ESR and immunoglobulins are not recommended screening tests for monoclonal bands, which are only detected by electrophoresis
Symptomatic patients
Although the diagnosis of multiple myeloma is often made by chance, a significant number of people will present with
symptoms.
Two-thirds of patients complain of bone pain, frequently located in the back, long bones, skull and pelvis. In addition,
patients often have a range of non-specific (constitutional) symptoms, including fatigue, weight loss, chronic infections,
paresthesia and symptoms related to hypercalcaemia.
| Table 3: Red flags for potential diagnosis of multiple myeloma in patients with back pain (adapted
from George, et al 1991)10 |
| Red Flags |
Age over 50 years
Pain that is worse in supine position
Pain that is worse at night or awakens patient from sleep
Pain with a band-like distribution around the body
Pain that is not relieved with conventional methods (i.e., rest, nonsteroidal anti-inflammatory drugs)
Associated constitutional symptoms (fever, weight loss, dehydration)
Progressive neurologic deficit in lower extremities |
|
As many patients with multiple myeloma present with lower back pain, a number of “red flags” have been identified
in the assessment of patients with acute lower back pain. Multiple myeloma should be considered as a diagnosis in patients
aged over 50 years with back pain persisting more than one month, if one or more red flags are identified (Table 3).
Testing for monoclonal gammopathy
In patients with a possible monoclonal gammopathy, the following investigations are required:
- Serum protein electrophoresis
- Urine free light chain testing
If a band is identified by serum electrophoresis or if immune paresis is noted then immunofixation, immunoglobulins
and band quantification are recommended. A casual urine sample for protein and albumin and free light chains (Bence-Jones
protein) should be collected. Other tests which should also be requested in this clinical situation are complete blood
count, corrected calcium, creatinine (eGFR) and electrolyte measurements.
Monitoring monoclonal gammopathy
Periodic monitoring and watching for clinical and laboratory features of change is of key importance when managing patients
with a monoclonal gammopathy. This is because transformation can occur, e.g. a patient may transition from MGUS to asymptomatic
myeloma to multiple myeloma.
Approximately 1% of people with MGUS develop multiple myeloma, amyloidosis or Waldenström’s macroglobinaemia
annually, although most (especially those who are older at diagnosis) die of other diseases.11
The follow-up for patients with MGUS depends on the risk of progression. Both the British/Nordic Study Group (2009)9 and
the International Myeloma Working Group (IMWG, 2010)12 have recently published guidelines. These differ in
detail, but their common thread is that it is important to fully investigate patients at high risk, whereas those with
low risk can be spared unnecessarily invasive initial investigations and need less frequent long-term monitoring.
The patient should be informed about the range of possible symptoms and advised to report new symptoms such as bone
pain, weight loss, fatigue or other symptoms of progression. They should be aware that the risk of progression is life-long
and does not plateau. The risk of eventual progression is higher for a young fit person with more years of life expectancy,
than for an older person with other significant co-morbidities.
Low risk MGUS: The majority of patients with MGUS are at low risk of progression, judged by:
- Small band size (IgG <15 g/L OR IgA or IgM <10 g/L)
- They are asymptomatic
- No other abnormal results (normal adjusted calcium, creatinine and eGFR, blood count).
These patients should be followed-up several times in the first year, and this interval can be extended to six to 12
monthly and up to two to three yearly in long-term stable patients.9,12
High risk MGUS: These patients should be referred to a haematologist and require more active initial
evaluation and closer long-term monitoring. Risk factors include one or more of the following:
- Band size IgG > 15 g/L OR IgA or IgM > 10 g/L
- Any IgD or IgE monoclonal gammopathy regardless of concentration
- Symptoms or signs of a suspected multiple myeloma or lymphoproliferative disorder, e.g. bone pain or pathological
fractures, constitutional symptoms such as weight loss, peripheral neuropathy, nephrotic syndrome
- Other unexplained laboratory or radiology abnormalities regardless of band size, e.g. hypercalcaemia, renal impairment,
anaemia, lytic lesions or significant osteopaenia
- The presence of Bence-Jones proteinuria, immune suppression, age and sex are not in themselves prognostic. However,
significant Bence-Jones proteinuria (>500 mg/L) should prompt haematologist referral because of the risk of development
or progression of renal impairment.
Further evaluation usually includes bone marrow aspirate and trephine biopsy. Serum free light chains and beta-2 microglobulin
may also be helpful in stratifying these patients – a normal light chain ratio and low beta-2 microglobulin level carries
lower risk.
Other possible investigations for higher risk patients include; spine/pelvic MRI scan for possible lytic lesions, abdominal
CT for retroperitoneal lymph nodes (in patients with IgM bands), and renal investigations including; possible renal/tissue
biopsy for patients with unexplained nephrotic proteinuria or renal impairment (looking for amyloidosis).
Long-term patients with a high risk MGUS should usually be monitored at least several times in the first year, then
annually for life. New symptoms or laboratory abnormalities should prompt earlier review. An increase in band size of
more than 25% over three months (minimal 5 g/L) is regarded as significant.
Patients with asymptomatic multiple myeloma have significant risk (about 10% annually) of progression
to symptomatic disease, and should be under haematologist review. A skeletal survey and a bone marrow aspirate and biopsy
should be carried out at baseline. Laboratory tests and clinical work-up should be done at diagnosis including baseline
MRI of the spine and pelvis. Tests should be repeated two to three months after the initial recognition of the diagnosis.
If the results are stable, the studies should be repeated every four to six months for one year and, if stable, evaluation
intervals can be lengthened to every six to 12 months. A skeletal survey should be performed if there is evidence of progression.
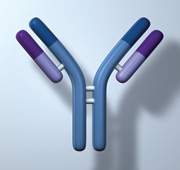
Understanding immunoglobulins
Plasma cells produce immunoglobulins which are composed of heavy and light chains. Each plasma cell produces only one
type of heavy chain (IgA, IgD, IgG, IgM and IgE) and one type of light chain (either kappa or lambda [κ or λ]).
After the chains are produced they are assembled within the plasma cell to form a whole immunoglobulin.
Figure 2: Immunoglobulin (showing light and heavy chains).