This year’s vaccine is different to previous years
It is too early to predict how severe the 2013 influenza season will be in New Zealand, however, a particularly severe
outbreak was seen in Canterbury in 2012 with a similar virus to that expected to be the dominant strain in all centres
this year. The Centres for Disease Control and Prevention (CDC) reported an earlier than normal start to the Northern
Hemisphere season with rapid increases in the rates of influenza-associated hospitalisations and deaths among older people
in the United States.1
Influenza vaccination during the New Zealand 2013 season is more important than in recent years because this year the
vaccine contains two new strains that were not present in the 2010 – 2012 vaccines. A key message for health professionals
to deliver to patients is, therefore, that: “there may be strains of the flu circulating this year that vaccinations from
previous years are unlikely to provide protection against.”
The decision to change the vaccine composition follows a World Health Organisation (WHO) recommendation, after laboratory
testing showed changes in antibody reactions to circulating strains and also a change in the dominant viruses in circulation.2
The 2013 vaccine contains:3
- A/California/7/2009 (H1N1)-like strain
- A/Victoria/361/2011 (H3N2)-like strain (new)
- B/Wisconsin/1/2010-like strain (new)
Two vaccines are funded for 2013:
- Fluarix is approved for use in adults and children aged over six months
- Fluvax is approved for use in adults and children aged over five years, however, Public Health experts recommend that
Fluvax should not be given to children aged under nine years and should not be given to children who have a history of
febrile convulsions. This follows research showing increased rates of febrile reactions, including febrile convulsions
in this age group.4
Neither vaccine should be administered to people who have had anaphylactic reactions to any of the vaccine’s components.3 Fluarix
contains traces of gentamicin sulphate and Fluvax contains traces of neomycin and polymyxin.3 Both influenza vaccinations
are derived from hen eggs and may contain residual egg protein. People who have had a confirmed anaphylactic reaction
to egg protein should only be administered the vaccine under specialist supervision.3 People who have a non-anaphylactic
allergy to eggs can be administered the vaccine as normal. People who are acutely unwell or have a fever over 38°C should
delay having the vaccine until they are well.
How many doses of the vaccine are needed?
One dose is required for adults, children aged over nine years, or children aged between six months and nine years who
have already received an influenza vaccination in any previous year. Children aged between six months and nine years who
are receiving their first influenza vaccination should have two doses, at least four weeks apart.7 This is
because they may not have had contact with viruses with antigenic properties similar to the strains present in the vaccine
and are likely to require an additional dose to establish immunity.
Who is eligible for subsidised vaccinations?
The following eligible groups will receive a fully subsidised seasonal influenza vaccination if they visit their General
Practice clinic before 31 July, 2013:
- Anyone aged 65 years and over
- Anyone with cardiovascular, cerebrovascular or chronic respiratory disease, cancer, Type 1 or 2 diabetes or a specified
condition
- Pregnant women at any stage of gestation
 A complete list of conditions that qualify a person for free vaccination is available
from: www.influenza.org.nz/?t=887
A complete list of conditions that qualify a person for free vaccination is available
from: www.influenza.org.nz/?t=887
N.B. People with asthma who do not require regular preventive medicines, those in remission from cancer, and people
with hypertension and/or dyslipidaemia without evidence of end-organ disease are not eligible for subsidised influenza
vaccination.
Who else should be encouraged to get vaccinated?
People with risk factors for exposure to influenza and its complications should also be encouraged to be vaccinated,
in particular children aged between six months and five years. Risk factors for influenza complications include:
- Pacific or Māori ethnicity
- Living in a low socioeconomic area or a crowded household
- Exposure to second-hand cigarette smoke
- Frequent illness
Women who intend to become pregnant during the influenza season and people travelling overseas, especially to the Northern
Hemisphere from October to May should also be encouraged to be vaccinated.
Healthcare workers are strongly recommended to be vaccinated because they have high exposure rates to influenza virus
and immunisation of healthcare workers may reduce patient morbidity.8 Endorsement of vaccination by healthcare professionals
can influence an individual’s decision to be vaccinated, even if they did not initially want to be.9 Influenza
vaccination is free to all staff employed by District Health Boards in New Zealand. In 2012, almost half of all employees
received an influenza vaccination. Rates were highest among doctors (57%) and lowest among midwives (37%).10

PHO Performance Programme – Influenza vaccination rates are decreasing
Influenza vaccination is a PHO Performance Programme (PPP) Indicator that accounts for 9% of the Performance funding;
3% for the total population and 6% for the high need population.5 High need populations include Māori and Pacific Peoples
and people living in Quintile 5 (most deprived) socioeconomic areas. The target is assessed by counting the enrolled
patients aged 65 years and over who have received an influenza vaccination during the most recent campaign (the numerator).
This number is then divided by the number of enrolled patients aged 65 years and over at the beginning of the most recent
campaign period (the denominator). Only vaccinations received by people aged 65 years and over are included in the PPP
results. PHOs that have a large number of people who decline offers of vaccination will therefore find it difficult to
meet the target.
The programme goal is for at least 75% of people aged 65 years and over at the end of the annual influenza vaccination
season to have received the influenza vaccine during the most recent campaign.5
The rate of influenza vaccinations has trended downwards over the past five years (Figure 1) for people aged 65 years
and over. The most recent data captured from July – September 2012 suggests that in 2013 rates may have stabilised, with
the national rate of vaccination dropping only slightly from 64.4% to 63.7%.6 However, this is more than 10% below the
programme goal of 75% coverage. It is important that the trend of declining influenza vaccination rates in New Zealand
is reversed. In 2012, no PHO achieved the target rate of 75%.
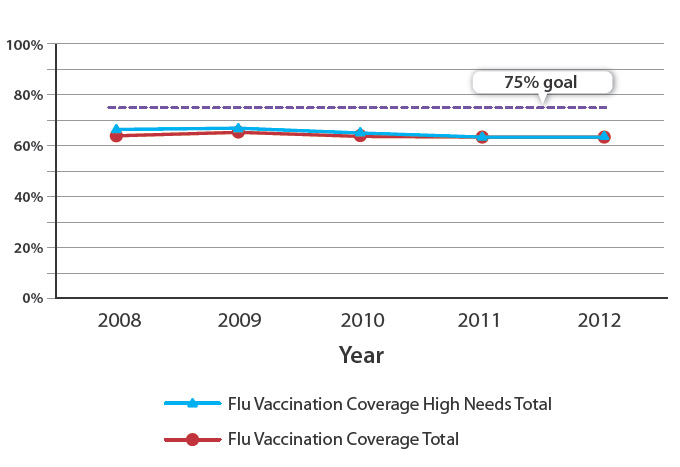
Figure 1: Influenza vaccination rates from 2008 to September 2012 for all people aged 65 years and over
in New Zealand5
Influenza vaccination is important for older people and women who are pregnant
Annual influenza vaccinations are important for people at increased risk of influenza-related complications. This is
not only because different strains of influenza may be in circulation each year, but also because antibody titre begins
to decline from one to two months post-vaccination.3 This is more pronounced in older people. Immunity levels
in people aged over 65 years have been shown to be significantly reduced at six months post-vaccination and may not be
sufficient to provide protection after this time.3, 11
Annual influenza vaccination may reduce cardiovascular risk. Mortality rates due to stroke and myocardial infarction
increase by 10 – 15% during winter.12 Recently, several studies have suggested that annual influenza vaccination
may have a cardio-protective effect. A meta-analysis, including over 290 000 patients, found that receiving an annual
influenza vaccination was associated with significant reductions in myocardial infarction (odds ratio 0.73), all-cause
mortality (odds ratio 0.61), and major adverse cardiac events (odds ratio 0.47).13 Another meta-analysis which
included over 30 000 participants aged over 55 years with known vascular disease, found that vaccination was associated
with a reduced risk of major vascular events when the virus in circulation was well matched to the vaccine.12
The pathophysiology of the possible effect of influenza on cardiovascular risk is not clear. Suggested mechanisms include
increased risk of plaque rupture, endothelial dysfunction, fever-associated tachycardia, impaired breathing, modulation
of blood clotting and immune and inflammatory processes.12, 13
Administering influenza vaccination during all trimesters of pregnancy is considered safe,14 and can be done
at the same time as pertussis vaccination occurs. Women who are pregnant and newborn infants are at increased risk of
influenza-related complications. Women with asthma or diabetes who are pregnant are three to four times more likely to
contract influenza and develop an influenza-associated illness.3 Neither Fluvax or Fluarix are approved for
use in infants aged younger than six months, however, young infants have high rates of hospitalisation from influenza.
Vaccination during pregnancy is therefore the best way to decrease a newborn infant’s influenza risk because it increases
antibody delivery, giving temporary protection to the infant via the placenta. Ideally all siblings and carers of infants
will also be vaccinated to provide a “cocoon of immunity” around the infant. Studies on the effectiveness of pertussis
vaccination have shown that immunisation of family members can provide protection to infants where there is a high prevalence
of disease within the community.15, 16 It is likely that these findings apply to other vaccine preventable
illnesses such as seasonal influenza.
Reducing the number of patients who decline influenza vaccination
When discussing influenza vaccination with patients who may be reluctant to be immunised, it is important to emphasise
the following points:
- There are two new strains of “the flu” in the vaccine this year, in recognition of changing circulating strains internationally
- Annual immunisation is likely to help to reduce the risk of an older person having a stroke or heart attack in the
future
- Immunisation helps to protect the families and friends of people who are immunised who may be more vulnerable to the
complications of influenza
Best Practice Tip: A standardised statement can be prepared for the practice to use when offering influenza vaccination
to patients. This may be of particular use when phoning patients who are unlikely to present for vaccination without
encouragement. The “Don’t let the flu get you!” website has template patient recall letters which may be useful when
contacting patients who have previously declined vaccination. Available from:
www.influenza.org.nz
Pneumococcal vaccination
Pneumococcal infection by the bacterium Streptococcus pneumoniae is a frequent cause of respiratory illnesses, e.g.
otitis media, bronchitis and sinusitis. Many people in New Zealand carry these bacteria without developing invasive disease.
However, serious complications such as pneumonia, meningitis and septicaemia can develop when S. pneumoniae invades normally
sterile tissue. Young children, older adults and people who are immunodeficient are most at risk of this occurring.
Four pneumococcal vaccines are licensed in New Zealand. Synflorix (10-valent) and Prevenar13 (13-valent) are conjugate
vaccines. Pneumovax23 and Pneumo23 (both 23-valent) are polysaccharide vaccines. Conjugate vaccines generate better quality,
more longer-lasting antibodies and have immune memory unlike polysaccharides. Conjugate vaccines are also more effective
when used as boosters.
The 10-valent Synflorix vaccine at age six weeks and age three, five and 15 months is funded for all infants as part
of the National Immunisation Schedule.17 Prevenar13 vaccine is used for children at high risk of complications,
followed by Pneumovax23 vaccine after age two years.17 High-risk children aged under five years (Table 1)
and all people with functional or anatomical splenectomy are eligible for fully subsidised vaccination with both Prevenar13
and Pneumovax23.
Pneumovax23 vaccination is recommended by the Ministry of Health, but not subsidised, for all people aged 65 years or
over and adults and children aged over five years at increased risk of invasive pneumococcal disease due to co-morbidity
or immunodeficiency (Table 1), who have not been previously immunised.17 The Immunisation Advisory Centre
also recommends that Prevenar13 be given eight weeks before Pneumovax23 in high-risk patients, to produce better immune
response.18 This is an ideal, but potentially expensive strategy for the patient.
Healthy people aged over 65 years generally require only a single dose of Pneumovax23, but those at high risk should
receive a second dose three to five years after their first dose.
There are no contraindications to pneumococcal vaccination other than a previous severe reaction to the vaccine or any
of its components. The safety of the vaccine has not been confirmed in pregnant women, therefore it is recommended that
immunisation occur following pregnancy, unless the risk of infection is substantial.17
 For further information see: “The management
of community-acquired pneumonia”, BPJ 45 (Aug, 2012).
For further information see: “The management
of community-acquired pneumonia”, BPJ 45 (Aug, 2012).
| Table 1: Children and adults considered to be at high risk of pneumococcal disease17 |
| Children with these conditions/treatments are considered high risk* |
Adults with these conditions/treatments are considered high risk |
- Receiving immunosuppressive or radiation therapy
- Primary immune deficiencies or HIV
- Renal failure or nephritic syndrome
- Diabetes
- Down syndrome
- Organ transplants
- Cochlear implants or intracranial shunts
- Cerebrospinal fluid leaks
- Receiving long-term corticosteroid treatment and daily prednisone, or taking ≥ 20 mg prednisone per day
- Pre-term infants born prior to 28 weeks gestation
- Chronic pulmonary disease, including asthma treated with high-dose corticosteroids
- Cardiac disease with cyanosis or failure
|
- Aged over 65 years
- People with a history of invasive pneumococcal disease
- Functional or anatomical asplenia, e.g. sickle cell disease or splenectomy
- Chronic illness, e.g. chronic cardiac, renal or pulmonary disease, diabetes or alcoholism
- Immunocompromised, e.g. nephritic syndrome, lymphoma and Hodgkin’s disease, HIV
- Cerebrospinal fluid leak
- Cochlear implants
|
| * Eligible for funded pneumococcal vaccination if aged under five years |
Acknowledgement
Thank you to Dr Nikki Turner, Director, CONECTUS and The Immunisation Advisory Centre, University
of Auckland and Associate Professor Lance Jennings, Virologist, University of Otago, Christchurch and
Canterbury Health Laboratories, Canterbury DHB for expert guidance in developing this article.