In this article

View / Download pdf version of this article
On Thursday, 19 July, 2012, the New Zealand Formulary (NZF) was officially launched by the Hon. Peter Dunne, Associate Minister of Health. The NZF is now available online, free of charge, to all healthcare professionals in New Zealand.
What is the NZF?
The NZF is an independent medicines information guide for healthcare professionals in New Zealand. The website is also accessible free of charge to the general public.
The NZF has been developed through a partnership between the Best Practice Advocacy Centre Inc (BPAC Inc), the Best Practice Advocacy Centre New Zealand (bpacnz) and the Royal Pharmaceutical Society of Great Britain, publishers of the British National Formulary (BNF).
The NZF is based on the BNF, but has been adapted for the New Zealand healthcare context. It has four main components:
- General notes on medicine use
- Practical guidance on specific therapeutic categories, e.g. cardiovascular, respiratory. Links to supporting local guidance are provided where appropriate.
- Detailed summaries (monographs) of individual medicines
- Details of preparations available and subsidy information (via linkage with the New Zealand Universal List of Medicine)
Sections are included on medicine use in pregnancy, breast feeding, renal disease and palliative care, as well as information on adverse medicine reactions and medicine interactions, incorporating advice and guidance from two reputable sources; Stockley's Interactions Alerts and the BNF. Information and links are provided to groups such as the National Pharmacovigilance Centre (for adverse drug reporting), Medsafe and the National Poisons Centre.
Figure 1 shows an example of a medicine monograph in the NZF. Monographs have been designed to allow maximum information within one screen, with "clickable" links to more detailed layers of information.
The NZF team is currently working on an adaption of the BNF for children (BNFc), to produce the first New Zealand Formulary for children (NZFc – available July 2013). In the meantime, where relevant, links are provided directly to the BNFc from the NZF text.
Figure 1: Example monograph
Table of Contents
This shows the hierarchical arrangement of documents in the NZF and is located on the home page, and the left-hand side of the web page. You can use the Table of Contents to navigate to any chapter or section within the NZF.
|
Search function
By clicking on the search NZF tab located on the top right hand corner of the web page, it is possible to search for a specific drug monograph for a particular drug (the left-hand side search box), or to search the entire formulary for a word, phrase or trade name (using the right-hand side search box).
|
Interactions search
By clicking on the interactions tab located on the top right hand corner of the web page, it is possible to search for interactions between medicines.
|
Interactions
Within a monograph, interaction information from both the Stockley's alerts database and BNF interactions summaries are provided.
|
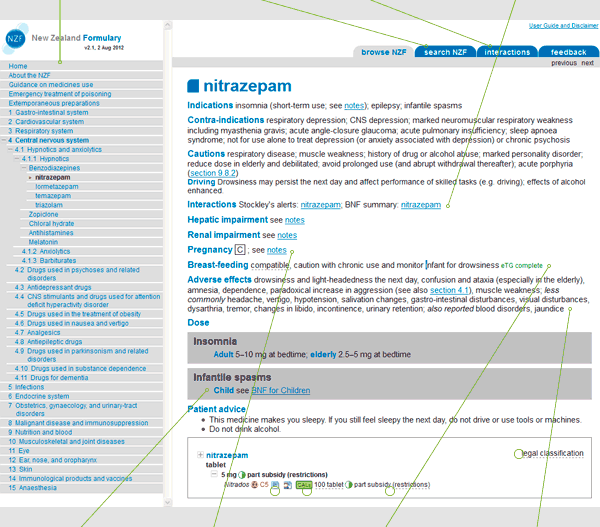 |
BNF for Children
Where appropriate links are provided to the relevant section of the BNF for children (BNFc).
|
Pregnancy
Where available the NZF classifies drugs according to the Australian categorisation system for prescribing medicines in pregnancy, 2011, Therapeutic Goods Administration.
|
Breastfeeding
Where available, the NZF drug monograph includes information on the drug's compatibility with breastfeeding as provided by Therapeutic Guidelines Limited.
|
Adverse effects
Adverse effects are reported as; very common (greater than 1 in 10) and common (1 in 100 to 1 in 10); less commonly (1 in 1000 to 1 in 100); rarely (1 in 10 000 to 1 in 1000); very rarely (less than 1 in 10 000); also reported frequency is unknown.
|
 Links and hovers
Links and hovers
Hyperlinks are shown as blue underlined text and lead you to additional relevant information in other parts of the NZF or to external web sites. Links to Medicines Data Sheets and patient information leaflets are also included.
|
How do I access it?
The NZF may be viewed online or downloaded as an eBook or PDF from the website. The NZF is publically accessible in New Zealand only. Future versions will also be available integrated within prescribing and dispensing software.
eBook formats include; ePub (universal file format for eReaders excluding Kindle), MOBI (for Kindle) and PDF (for eReaders and computers). Full instructions on downloading the NZF eBook are available from the website.
The NZF can also be accessed within Medtech via an on-screen icon.
How often is it updated?
The information in the NZF is continually revised by the NZF team and a panel of experts. Updates to the online and eBook versions of the NZF are published monthly, on the first working day of each calendar month. Users may register for email notification of eBook updates.
History of the NZF
The concept of a national medicines formulary for New Zealand was first discussed by key players in the NZF partnership, more than twenty years ago. As a result of discussions, the Department of Health issued a "request for proposals" for a national formulary in 1992. The Department envisaged a publication similar in style and content to the British National Formulary, which was provided annually to General Practitioners, but with emphasis on a New Zealand specific context. Unfortunately the funding for the formulary was withdrawn before a contract was established and no further official progress was made on a national formulary for New Zealand for a number of years.
In the meantime, BPAC Inc was established and, as one of its key projects, it began work on investigating the concept of developing a national formulary. The Royal Pharmaceutical Society granted permission for BPAC Inc to access text and editing tools from the BNF, and as a test, one chapter was successfully adapted to New Zealand requirements.
Between 2001 and 2006, the WHO Medicines Strategy, the Ministry of Health's WAVE advisory group and Health Information
Strategy and reports from the University of Auckland and BPAC Inc all concluded that a national medicines formulary
would be beneficial for New Zealand. DHBNZ, with support from PHARMAC, developed a business case for a national medicines
formulary in 2007, which garnered considerable support from the health sector. Funding was allocated in the 2008 Budget
for the development of a national formulary, as part of the New Zealand Medicines Strategy. The Hon. Peter Dunne, Associate
Minister of Health, was key in securing this funding, based on consistent feedback he received from healthcare professionals
concerning the lack of robust, New Zealand specific medicines information.
"Excellent website. Extremely useful for study and looking forward to using it as a Pharmacist. Everything I need in one, easy to use website, thank you"
"NZF is AWESOME! Thank you! Very exciting!"

The New Zealand Universal List of Medicines (NZLUM) was released in 2010, as a foundation for a national formulary. The NZLUM Steering Group was then charged by the Ministry of Health to establish, what they provisionally titled, the New Zealand Medicines Formulary (NZMF). A request for proposals for the provision of the NZMF was issued in December 2010. Following a detailed evaluation and competitive process, the contract for a national formulary was awarded to the New Zealand Medicine Formulary Limited Partnership (NZMF LP) in September 2011, a collaboration between BPAC Inc, bpacnz and the Royal Pharmaceutical Society.