In this article

View / Download pdf version of this article
Finding information about medicines can often be an arduous process – there is no shortage of information both in hard copy and online, but how do you know which source to trust and how do you go about finding information that is New Zealand specific? What about subsidy information? What about medicines information that will help in the management of patients with more complex or long-term illnesses? What about interactions?
In September 2011, after a "request for proposal" process, the Ministry of Health announced that the New Zealand Medicines Formulary Limited Partnership (NZMF LP) was the preferred provider of a medicines formulary for New Zealand. The NZMF LP is a partnership between Dunedin-based bpacnz and BPAC Inc, in conjunction with the Royal Pharmaceutical Society (United Kingdom).
The development of the NZF is on track for full public release on 19 July 2012.
About the NZF
The NZF is a resource that will be available free of charge for all healthcare professionals prescribing, dispensing and administering medicines across community and hospital care. A true "one stop shop", the NZF addresses the need for general purpose, point-of-care information about the use of medicines in New Zealand. It will aid in decision making and contribute to best practice through standardised and evidence-based information about medicines. Over time the NZF will be fully integrated into the e-health environment, including prescribing and dispensing systems across primary and secondary care.
The NZF builds on the New Zealand Universal List of Medicines, and incorporates information from the British National Formulary (BNF). It is adapted for the New Zealand context and covers medicines used in New Zealand, including Section 29 medicines where appropriate.
Initially, the NZF will cover information such as:
- Medicine indications, dosage, cautions, contraindications, side effects, warnings, patient advice and cautionary and advisory labelling
- The use of medicines in renal and hepatic impairment, pregnancy, lactation and sport
- Subsidy information
- Medicine interactions
- Concise disease management advice
- Adverse event reporting
Further enhancements are planned over time such as the development of the New Zealand Formulary for Children, tools to allow integration of preferred medicines lists and local protocols in hospital care, and other extensions according to user feedback.
When publically released on 19 July 2012, the NZF will be available:
- In a format ready for integration into clinical IT systems used by general practices, hospitals and community pharmacy. The NZF team are currently working with IT vendors to ensure that integration of the NZF into clinical IT systems occurs as soon as possible.
- As an application for installation on individual computers
- As an eBook
- To third parties for the development of added value applications, e.g. smart phones and tablet computers
- Online at: www.nzformulary.org
Governance
The NZMF LP Board has representation from each of the three partners. The Chief Executive Officer is Professor Murray Tilyard. There are two advisory groups that assist the clinical editorial team – the New Zealand Formulary Advisory Board (NZFAB) and the Editorial Advisory Board (EAB).
The NZFAB is the representative body that advises on the NZF product from a sector/clinical user perspective. It is chaired by Dr Don Mackie, Chief Medical Officer, Ministry of Health. Each member of the NZFAB has a responsibility to liaise with their representatives to gain a thorough understanding of sector needs and ensure that the NZF continues to meet these needs.
The EAB is responsible for reviewing the clinical content of the NZF. It is chaired by Professor John Campbell, Professor of Geriatric Medicine, University of Otago and consultant physician, Dunedin Hospital, Southern DHB. The responsibility of the EAB is to ensure that the content is clinically sound, of high standard and relevant to New Zealand practice. The EAB receives advice and guidance on policy and scope of content, but is independent of the NZFAB with respect to editorial and clinical processes.
The clinical editorial team
The clinical editorial team is comprised of managing Editor and clinical pharmacist, Dave Woods, and his Dunedin-based team of five clinical pharmacists, supported by advice from external medical specialists and associate editors.
The clinical editorial team has made excellent progress in reviewing, and customising to the New Zealand context, 19 chapters of the BNF, containing more than 1000 medicine monographs and associated prescribing notes. The team is on track to complete this significant piece of work by the end of May 2012, in time for the formal release of the NZF on 19 July 2012.
 For further information, email: contact@nzformulary.org
For further information, email: contact@nzformulary.org
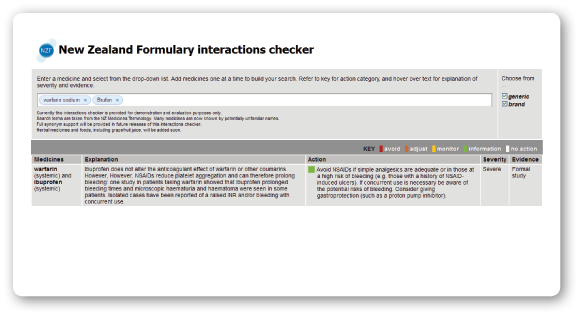
One of the early deliverables for the NZF is an online interactions checker. For a sneak preview, visit: www.nzformulary.org
Have a go and tell us what you think!