For updated screening recommendations, see:
"The
annual diabetes review: screening, monitoring and managing complications"
In this article 
View / Download
pdf version of this article
| Key concepts |
- Sight-threatening diabetic retinopathy is largely preventable through regular retinal screening and prompt treatment
- Retinal screening should be carried out at least every two years. More frequent screening regimens are indicated
by clinical risk factors such as the duration of diabetes and the degree of pre-existing retinopathy.
|
- Primary care plays a critical role in ensuring that patients are referred for and attend retinal screening so they
can be treated before there is visual deterioration
- Maintaining good glycaemic control, treating hypertension and managing lifestyle risk factors, especially smoking
cessation, is also essential
|
"Get Checked" for diabetes complications
Approximately 5 – 7% of New Zealand adults have been diagnosed with type 1 or type 2 diabetes.1 The
actual number of people with diabetes is likely to be much higher than this. The self-reported prevalence of diabetes
is two to three times higher among Pacific, Māori and Indo-Asian people.1 Diabetes is a leading cause
of blindness, end stage kidney failure and complications leading to lower limb amputation. It is a major risk factor for
cardiovascular disease and early mortality.
Regular health checks are essential to reduce the frequency of complications from diabetes, as well as to minimise their
impact. The “Get Checked” programme is a national initiative, offering free annual health reviews to people
with diabetes, by their GP or practice nurse. The programme aims to promote early detection and intervention for problems
associated with diabetes.
The “Get Checked” annual health review includes:
- A HbA1c level
- Cardiovascular risk assessment, including blood pressure, lipid profile, height and weight
- Kidney function (microalbuminuria)
- Sensation and circulation of feet
- Retinal check (at least every two years)
- Follow-up plan for care
The annual check for people with diabetes is also a PHO Performance Programme (PPP) indicator.
The PPP goal is for at least 80% of all people with diabetes enrolled in a practice to have had a full annual “Get
Checked” review each year.
In 2009 53% of the estimated number of people with diabetes in New Zealand had an annual “Get Checked” review.2 This
is an improvement from the previous year (46%),2 but this number still falls considerably short of the PPP
target of greater than 80%.
Between 2008 and 2009 annual reviews in the high needs population (identified as Māori and Pacific peoples and
those living in lower socioeconomic areas) improved from 52% to 57%.2
There is much variation throughout DHB regions and PHOs, with some areas achieving better results. Technical difficulties
in data collection may contribute to lower figures in some areas.
Consider the barriers to achieving this goal and ways in which the practice can address this. People with diabetes who
are not receiving an annual free review, are potentially at a greater risk of developing harm from complications, which
could have been treated if detected early enough.
Diabetic retinopathy is one of the leading causes of blindness in New Zealand
Diabetic retinopathy has been, until recently, the leading cause of preventable adult blindness and vision impairment
in New Zealand. Factors such as advances in treating retinal damage and more effective screening are slowly decreasing
the prevalence of diabetic retinopathy in some areas.
The exact incidence of diabetic retinopathy is unknown but it is estimated that 30% of people with diabetes have some
degree of retinopathy, with 10% having sight-threatening retinopathy.3 A New Zealand study of almost 12,000
people with diabetes, conducted between 2003 and 2005, found that almost one-third (32%) had some signs of diabetic retinopathy.4 There
was also evidence that Māori were accessing the retinal screening service at a lower rate than other ethnic groups.4 As
this study was based in one particular region of New Zealand (Wellington), incidence of diabetic retinopathy and disparities
in accessing services, may be even greater in other areas. An earlier small study of almost 500 people with type 2 diabetes
in South Auckland found that the prevalence of moderate to severe retinopathy was 4% in Europeans, 13% in Māori and
16% in Pacific peoples.5
The longer the duration of diabetes, the greater the prevalence of retinopathy. A large longitudinal study, based in
the United Kingdom, found that the incidence of sight-threatening diabetic retinopathy after five years, in patients with
diabetes (type 1 or 2) who had no signs of retinopathy at baseline, was 3.9%. In patients who initially had mild diabetic
retinopathy, 15% had developed sight-threatening retinopathy by five years.6
Sight-threatening diabetic retinopathy is largely preventable, through regular retinal screening and prompt treatment.
Primary care plays a critical role in ensuring that patients are referred for and attend retinal screening so they can
be treated before avoidable loss of vision occurs.
Detecting and preventing diabetic retinopathy
Diabetic retinopathy is asymptomatic until it is at an advanced stage and then it is usually too late for effective
treatment. Therefore early detection and prevention are imperative.
In primary care the two key responsibilities are:
- Referral for regular retinal screening at least every two years (and following-up on attendance and subsequent treatment
if needed)
- Management of risk factors
Early detection of retinopathy with regular screening can save vision
The objectives of retinal screening in people with diabetes are to:3
- Screen those with known diabetes for the onset of diabetic retinopathy
- Identify those with early microvascular disease so primary care and diabetes teams can optimally manage risk factors
such as glycaemic control and hypertension
- Refer those with more significant retinopathy who are at risk of visual impairment for management and treatment by
an ophthalmologist, before avoidable loss of vision occurs
N.B.: People with pre-diabetes (impaired glucose tolerance and impaired fasting glucose) do not require retinal screening
and should not be referred.
Referral process for screening
- Make a referral to the local retinal screening provider
- Check with the patient at their next consultation, that they have been assigned an appointment time for retinal screening
(or they have attended the appointment) and follow-up with the provider if this has not occurred
- Request and review a copy of the screening results, ensure that appropriate follow-up has occurred e.g. check that
referral to an ophthalmologist has occurred if indicated, or make a note in the patient record that a more frequent screening
interval has been recommended
- Place an automatic recall on the patient’s notes for when screening is next due
- Follow-up patients who do not attend for screening, ask them what their difficulties in attending are, consider barriers
to screening and how your practice may help address these
Each DHB has an individual arrangement with local providers for retinal screening (contact your local DHB if you are
unfamiliar with referral options). Screening is usually performed by a suitably trained optometrist, photographic technician,
ophthalmologist or other clinician. A designated ophthalmologist usually oversees each local retinal screening programme,
to ensure consistency in grading of retinopathy.
Some areas may be under-resourced for the numbers of patients who require retinal screening. In some cases, if the public
waiting list is too long, patients may be referred privately. A new study, soon to be published, suggests that the waiting
time for referral to an ophthalmologist for moderate background retinopathy or mild maculopathy varies considerably throughout
the country, but in most
cases* is less than the recommended referral time for this grade of disease (four to six months).7
Do not wait for signs and symptoms to occur
Early retinopathy is asymptomatic. Signs of blurred or fluctuating vision, spots or “floaters”, if related
to diabetic retinopathy, are most often associated with advanced disease.
People with diabetes who present with an acute impairment of vision from any cause should be referred for urgent review
with an ophthalmologist/eye clinic.
 Best practice tip: Retinal photo-screening for diabetic retinopathy
does not constitute a full eye examination. Patients should still be regularly reviewed for other eye pathologies such
as cataracts or glaucoma. Primary care clinicians can test visual acuity using an eye chart and pinhole. As a
general rule, if visual acuity improves with pinhole testing, then it is more likely that any reduction in visual acuity
is due to a refractive error (and may require subsequent referral to an optometrist) rather than due to pathology in the
eye (which would require referral to an ophthalmologist).
Best practice tip: Retinal photo-screening for diabetic retinopathy
does not constitute a full eye examination. Patients should still be regularly reviewed for other eye pathologies such
as cataracts or glaucoma. Primary care clinicians can test visual acuity using an eye chart and pinhole. As a
general rule, if visual acuity improves with pinhole testing, then it is more likely that any reduction in visual acuity
is due to a refractive error (and may require subsequent referral to an optometrist) rather than due to pathology in the
eye (which would require referral to an ophthalmologist).
Screening intervals
New Zealand guidelines recommend that retinal screening is carried out every two years for a person with diabetes who
does not have retinopathy (Table 1).3
| Table 1: Summary of screening recommendations for diabetic retinopathy3 |
| |
First retinal screen |
Screening interval: no retinopathy |
Screening interval: retinopathy detected |
Type 1 diabetes
|
Five years after diagnosis or after puberty |
Two-yearly |
More frequent than two-yearly, determined by severity, glycaemic control and other
risk factors |
| Type 2 diabetes |
Soon as possible after confirmed diagnosis
|
Two-yearly |
Pregnancy + diabetes
|
First trimester of pregnancy |
Two-yearly |
Frequent throughout pregnancy (also if poor glycaemic control, even if no retinopathy) |
A referral for screening should be made at the time of a confirmed diagnosis for people with type 2 diabetes because
many people already have some degree of retinopathy at this stage. With type 1 diabetes, vision threatening retinopathy
is very rare in the first five years after diagnosis or before puberty so screening may commence after this time.3
Clinical factors that may affect screening intervals
In some circumstances, risk factors may be present indicating that earlier re-screening or referral to an ophthalmologist
should be considered.
These factors include:3
- Poor compliance – failure to attend appointments for screening on two or more occasions
- Poorly controlled diabetes – HbA1c > 75 mmol/mol (> 9%)
- Duration of diabetes – including type 1 diabetes for greater than seven years
- Rate of progression of retinopathy
- Insulin treatment in people with type 2 diabetes
- Poorly controlled hypertension
- Renal failure
- Ethnicity – Māori, Pacific and Asian peoples are at a higher risk of complications of diabetes
- Asymmetrical disease – i.e. significant worsening in one eye
For people with diabetes who have early signs of retinopathy, screening should be more frequent. The frequency of screening
is determined by the Guidelines and the clinician’s opinion, taking into consideration factors such as the severity
of the retinopathy, glycaemic control, blood pressure and the risk of progression (see sidebar).3
Diabetic retinopathy can progress rapidly during pregnancy. Women with diabetes who become pregnant should be screened
in the first trimester of their pregnancy. If no retinopathy is detected and the diabetes is well controlled the two-yearly
screening schedule may be continued. If a minimal degree of retinopathy is detected or if the diabetes is not well controlled,
three-monthly screening for the remainder of the pregnancy is recommended. Referral to an ophthalmologist is required
if more than minimal retinopathy is detected.3 N.B. Women who develop gestational diabetes during pregnancy
are not generally at increased risk of retinopathy unless they have pre-existing disease.
 Copies of the Ministry of Health retinopathy screening guidelines and a CD for
training purposes can be obtained from:
www.moh.govt.nz/
Copies of the Ministry of Health retinopathy screening guidelines and a CD for
training purposes can be obtained from:
www.moh.govt.nz/
Retinal screening methods
The current “gold standard” method for screening for diabetic retinopathy in New Zealand is digital photography
of the retina while the pupil is dilated. Non-mydriatic photography is however widely used and, although not suitable
for every patient, does avoid the inconvenience of pupil dilation. If retinal photography is unavailable the fundus (interior
surface of the eye) can be examined through a dilated pupil using slit-lamp biomicroscopy. An assessment of visual acuity
should also be carried out.3
Conventional retinal examination involves using an ophthalmoscope to view the fundus through a dilated pupil, in a darkened
room. However it is difficult for even the most experienced examiners to achieve high sensitivity of retinopathy detection
with this method. Macular oedema is also not generally able to be detected with an ophthalmoscope.
After screening, the examiner grades the degree of retinopathy in each eye and applies an overall grading, depending
on the worst affected eye. It is important that the examiner follows standardised New Zealand screening protocols for
grading.3 The grade of retinopathy determines what follow-up action is taken.
Fluorescein angiography can be used to detect macular oedema if this is suspected. This involves dye being injected
into the arm and img taken as the dye progresses through the blood vessels in the retina.
Pupil dilation
Retinal examination usually involves pupil dilation using tropicamide 1% eye drops. This is safe for most people with
diabetes, including those being treated for chronic open angle glaucoma (but not closed angle glaucoma).
Patients should be warned that pupil dilatation may temporarily cause:3
- Distorted vision
- Disturbance of balance
- Lack of tolerance to bright light or sunlight – advise patients to take sunglasses with them to their clinic
appointment
- Impairment in driving or using machinery – advise patients to arrange transport to and from their appointment
and to avoid driving or using machinery for several hours afterwards (until vision returns to normal)
In extremely rare cases, dilation of the pupil can cause acute closed-angle glaucoma. This occurs because the iris
is pushed into the angle (the junction of the iris and the cornea), blocking drainage and increasing intraocular pressure,
which in turn can damage the optic nerve. Symptoms include pain, redness and decreased vision in the affected eye, coloured
halos around lights, headache, nausea and vomiting. Acute closed-angle glaucoma is an emergency situation but is usually
able to be treated using a regimen of ocular drugs and laser treatment.8
Management of risk factors for diabetic retinopathy
The duration of diabetes is the most significant risk factor for diabetic retinopathy.3,6,9 Poor glycaemic
control is also a major contributor to both the risk of development and progression of diabetic retinopathy.3 Other
modifiable risk factors include hypertension and nephropathy.3 Elevated blood lipid levels have a weaker association
with diabetic retinopathy but contribute to overall cardiovascular risk in a patient with diabetes.
If a person with diabetes is found to have signs of mild retinopathy, managing risk factors can help prevent more advanced
changes from developing.
To reduce the risk of progression of diabetic retinopathy, focus on:
- Maintaining good glycaemic control – establish an individualised HbA1c target (see
here)
- Managing hypertension – New Zealand cardiovascular guidelines recommend reducing blood pressure to < 130/80
mm Hg for people with diabetes,10 however this level may not be achievable for some people. In the presence
of microalbuminuria or renal disease more aggressive control may be required to reduce blood pressure to < 125/75 mm
Hg.10
- Advising on management of lifestyle factors, especially smoking cessation and promoting exercise and a healthy diet
- Reducing blood lipid levels as part of overall cardiovascular health – aim for a reduction towards the target
level of total cholesterol < 4.0 mmol/L,10 although this level may not always be achievable (see
here)
Intensive glycaemic control
Factors that worsen the general prognosis for people with diabetes also worsen diabetic retinopathy. Intensive glycaemic
control has been found to reduce the rate of progression of diabetic retinopathy. The Action to Control Cardiovascular
Risk in Diabetes (ACCORD) study placed people with type 2 diabetes on either intensive glycaemic control (target HbA1c level
of <6.0%) or standard treatment (target HbA1c level of 7.0 – 7.9%). After four years, diabetic retinopathy
had progressed in 7.3% of people on intensive glycaemic control compared with progression in 10.4% of people on standard
therapy (P = 0.003).12
Intensive glycaemic control appears to reduce the risk of most complications of diabetes, including retinopathy but
it has to be balanced against the increase in risks, such as severe hypoglycaemia, and some concerns about increased risk
of mortality.
 See article “HbA1c targets in people with
type 2 diabetes – do they matter” for more information about intensive glycaemic control, including discussion
of risk factors and adverse effects.
See article “HbA1c targets in people with
type 2 diabetes – do they matter” for more information about intensive glycaemic control, including discussion
of risk factors and adverse effects.
Hypertension as an independent risk factor
Hypertension is an independent risk factor for visual degradation. Over time, hypertension directly damages the retina,
choroid and optic nerve and can result in events such as retinal vein and artery occlusion, retinal emboli, ischaemic
optic neuropathy, glaucoma and age related macular degeneration. People with diabetes and poor blood pressure control
are at an increased risk of progression of diabetic retinopathy.13
Aspirin and retinopathy
Many patients with diabetes and a high cardiovascular risk will be taking aspirin. If the patient also has retinopathy,
should aspirin be continued?
The Early Treatment Diabetic Retinopathy Study found that 650 mg aspirin per day had no effect on the progression of
retinopathy or the development and duration of vitreous haemorrhage. It was concluded that aspirin is not beneficial
for the treatment of retinopathy, but there is normally no contraindication to its use in patients with retinopathy,
when required for cardiovascular disease or other indications.11
Management of hypertension is essential for people with diabetes, however there is conflicting evidence as to whether
intensive control is beneficial for reducing progression of diabetic retinopathy, and what blood pressure level is required.
The UK Prospective Diabetes Study (UKPDS) found a 34% reduction in the risk of progression of retinopathy after nine years
in patients with tight control of blood pressure (target < 150/85 mmHg, using captopril or atenolol).14 However,
the later ACCORD study found no significant benefit for diabetic retinopathy progression in those on intensive hypertensive
control (< 120 mmHg, 10.4%) compared with standard treatment (<140 mmHg, 8.8%), nor a benefit in cardiovascular outcomes.12 The
differing parameters for intensive blood pressure control and time frames of these studies may have contributed to the
different conclusions.
Intensive lipid control
Optimal lipid levels are advised in people with diabetes for cardiovascular risk reduction, but the beneficial effect
of intensive lipid control on diabetic retinopathy is less clear, and there is little or no evidence that statins have
any benefit for retinopathy.
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, which involved patients from New Zealand,
Australia and Finland showed that there was a beneficial reduction in microvascular complications in people with type
2 diabetes taking fenofibrate for lipid control.15 Researchers found that laser therapy for retinopathy was
needed more frequently in people with poorer glycaemic control or blood pressure control, but plasma lipid concentrations
were not associated with the need for laser treatment. Patients on fenofibrate therapy (3.1%) had a reduced need for laser
treatment for retinopathy compared to placebo (14.6%), but only if they had a degree of pre-existing retinopathy before
lipid-lowering treatment commenced (P = 0.004). There was no significant difference in the need for laser treatment between
people taking fenofibrate (9.6%) and placebo (12.3%) overall (P = 0.19) or in the subset of patients without pre-existing
retinopathy (11.4% fenofibrate group vs. 11.7% placebo, P = 0.87). Researchers concluded that fenofibrate appears to reduce
the need for laser treatment for diabetic retinopathy, but that this mechanism was not related to plasma concentrations
of lipids.15
The recent ACCORD study found that diabetic retinopathy had progressed in 6.5% of people on intensive dyslipidaemia
therapy (160 mg daily of fenofibrate plus simvastatin) compared with 10.2% on standard therapy (simvastatin plus placebo)
(P = 0.006).12
Fenofibrate is not currently registered in New Zealand or funded on the pharmaceutical schedule. It is in the fibric
acid derivative class of drugs which also includes bezafibrate (registered and funded) and gemfibrozil (registered). Statins
remain the primary agent for lipid control in people with type 2 diabetes.
Getting help with reduced vision
Community organisations and agencies such as the Royal New Zealand Foundation of the Blind offer information about
low vision counselling, training, and other special services for people with vision impairments.
For further information visit: www.rnzfb.org.nz
Other micro- and macrovascular complications
There is a strong association between diabetic retinopathy and other microvascular complications such as nephropathy
and neuropathy, and all are associated with a higher cardiovascular risk. Managing risk factors for retinopathy will generally
also decrease the risk of these other complications of diabetes.
A person with coexisting retinopathy, renal and foot disease is at a high cardiovascular risk as well as high risk for
requiring amputation.
Diabetic “foot-eye” syndrome is a distinct set of symptoms that has been observed in people, usually with
type 2 diabetes. It is characterised by:16
- A foot ulcer attributable to peripheral neuropathy
- Diabetic retinopathy
- Self neglect, indifference towards illness
Patients who present with “foot-eye” syndrome have a poor prognosis due to underlying severe cardiovascular
disease.16
Genetic factors may also play a role
Despite good diabetic control and management of risk factors, diabetic retinopathy still progresses in some people.
Conversely, some people with uncontrolled risk factors do not progress to diabetic retinopathy. Genetic factors are thought
to play a role in the susceptibility to diabetic retinopathy.17
Characteristics of diabetic retinopathy
What causes diabetic retinopathy and how does it manifest?
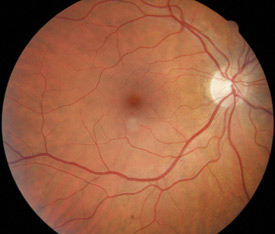
Above: A normal retina |
The microvascular changes that occur throughout the body as a result of diabetes, also affect the eye. Microvascular
changes within the retina are the most likely to adversely affect vision.
Diabetic retinopathy can be classified as non-proliferative (background) or proliferative. Visual loss and blindness
occurs via two different mechanisms – macular oedema and proliferative retinopathy. Macular oedema can occur at
any stage of diabetic retinopathy and is caused by blood vessel leakage and retinal thickening, due to breakdown of the
blood-retinal barrier. It is the most frequent cause of vision loss in people with type 2 diabetes. Proliferative retinopathy
occurs as a result of vascularisation induced by ischaemia, causing loss of vision due to haemorrhage or retinal detachment.18
Non-proliferative retinopathy
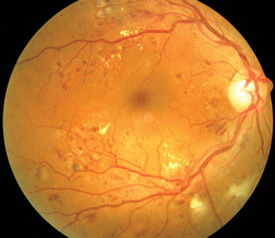
Above: Retina showing signs of retinopathy
including exudates and haemorrhages3 |
Lesions include:
- Microaneurysms – small swellings that form on small retinal blood vessels; if the swellings leak or burst,
plasma or blood can leak into the adjacent tissue.
- Haemorrhages – leakage of blood from the small vessels into adjacent tissues; haemorrhages deeper in the retinal
tissue (dot and blot) are more common in diabetes, superficial haemorrhages (flame) in the nerve fibre layer are more
common with hypertension
- Hard exudates – leakage of serum proteins and lipids caused by breakdown of the blood-retina barrier; appearing
as white or yellow crystalline deposits in the retina, sometimes forming a circular pattern around a leaking microaneurysm
- Cotton wool spots – formed from an accumulation of axoplasm due to occlusion of pre-capillary arterioles; appearing
as soft, fluffy, white lesions, often at right angles to the direction of the nerve fibre layer
- Macular oedema – damage to the central vision caused by functional damage and necrosis of retinal capillaries;
the leading cause of visual impairment in people with diabetes
- Venous loops and beading – damage to vessel walls resulting in formation of loops or beading (sausage shaping)
of the blood vessels; often a sign that more severe proliferative retinopathy is developing
Proliferative retinopathy
- Restricted blood supply to the retina (retinal ischaemia) caused by diabetes, can lead to the release of Vascular
Endothelial Growth Factor (VEGF) which initiates the formation of new blood vessels (neo-vascularisation)
- The new blood vessels break through and grow along the surface of the retina, the posterior hyaloid face (the wall
between the retina and the vitreous cavity inside the eye) and in severe cases, the iris
- The new blood vessels are fragile and easily broken, leading to haemorrhage in the vitreous cavity or pre-retinal
space, compromising vision almost immediately
- Over time, as the density of the newly formed blood vessels increases, fibrous tissue is formed which can adhere
to the retina and posterior hyaloid face of the eye
- The traction between the vitreous and the fibrous tissue connections can cause retinal oedema, tears and detachments
Symptoms
Early to moderate stages of diabetic retinopathy are usually without any noticeable symptoms. Symptoms may be experienced
in proliferative retinopathy, particularly with haemorrhage and retinal detachment.
Vitreous haemorrhage occurs suddenly but is not usually associated with any pain. The blood which enters the vitreous
cavity occludes the vision and is seen as spots or areas of visual loss. If not treated, repeated haemorrhages result
in progressive visual loss in most cases.19
Retinal detachment is described as flashing lights and floating spots in the peripheral vision or as a “curtain” progressing
across the visual field.20
NB: Sudden onset of visual symptoms or deterioration requires referral for specialist assessment, rather than for routine
screening for retinopathy.
How is diabetic retinopathy treated?
Laser photocoagulation is the primary treatment for sight-threatening diabetic retinopathy, however it is not always
completely successful in restoring visual loss. Better results are achieved if treatment is carried out earlier in the
disease process.18 For the majority of cases the goal of treatment with laser photocoagulation is to reduce
the rate of visual loss or to stabilise visual acuity.
During laser photocoagulation, a laser is applied to the retina, causing burns. Different methods of laser surgery
(or combinations of methods) are carried out depending on the pathology being treated.
Surgery is usually completed in one session, but if both eyes require treatment, this normally occurs several weeks
apart.
Adverse effects and complications of laser photocoagulation can include:
- Headache – usually relieved with rest and simple analgesia, but if severe or persistent, glaucoma must be ruled
out
- Pain – anaesthetic drops are applied, but an uncomfortable stinging sensation can occur as time progresses
- Blurred vision (temporary)
- Visual field restriction
- Decreased contrast sensitivity
- Impaired night vision or colour vision
Vitreous surgery
Vitreous surgery may be required in patients with some types of retinal detachment, vitreous haemorrhages and severe
proliferative retinopathy. Vitreous surgery has the potential for serious complications including significant visual
loss and eye pain.
A vitrectomy is performed under local or general anaesthesia. A tiny incision is made in the eye and the vitreous gel
clouded by blood is replaced by a saline solution. After the procedure the eye may be red and sensitive, an eye patch
may be required for a few days or weeks to protect the vision, and antibiotic eye drops may be required to reduce the
risk of infection.
Intravitreal corticosteroids
If laser photocoagulation has been unsuccessful, in some cases patients may be trialled on corticosteroids, injected
into the vitreous of the eye. This method can be effective, but re-injections are usually needed and there is a potential
for significant adverse effects such as infection, glaucoma and cataract formation.18
Acknowledgement
Thank you to Dr Paul Drury, Clinical Director, Auckland Diabetes Centre and Associate Professor
Gordon Sanderson, Ophthalmology, Dunedin School of Medicine, University of Otago for expert guidance in developing
this article.
References
- Ministry of Health (MoH). A portrait of health: key results of the 2006/2007 New Zealand Health Survey. Wellington:
MoH, 2008. Available from: www.moh.govt.nz (Accessed August, 2010).
- District Health Boards New Zealand (DHBNZ). Reporting back to the public - DHB Performance results June 2009. Wellington:
DHBNZ, 2010. Available from: www.dhbnz.org.nz (Accessed August,
2010).
- Ministry of Health (MoH). National diabetes retinal screening grading system and referral guidelines 2006. Wellington:
MoH, 2008. Available from: www.moh.govt.nz (Accessed August, 2010).
- Frederikson L, Jacobs R. Diabetes eye screening in the Wellington region of New Zealand: characteristics of the enrolled
population (2002 - 2005). N Z Med J 2008;121(1270).
- Simmons D, Clover G, Hope C. Ethnic differences in diabetic retinopathy. Diabet Med 2007;24(10):1093-8.
- Younis N, Broadbent D, Vora J, Harding S. Incidence of sight-threatening retinopathy in patients with type 2 diabetes
in the Liverpool Diabetic Eye Study: a cohort study. Lancet 2003;361:195-200.
- Hutchins E, Sanderson G, Rahman A, et al. Factors affecting effective implementation of the National Diabetes Retinal
Screening grading system and referral guidelines: A multi centre analysis: Unpublished report to the Health Research
Council New Zealand (pers comm G. Sanderson).
- Merck. The Merck manual for healthcare professionals; Angle-closure glaucoma: Merck, 2008. Available from:
www.merck.com/mmpe/sec09/ch103/ch103c.html (Accessed
July, 2010).
- Cikamatana L, Mitchell P, Rochtchina E, et al. Five-year incidence and progression of diabetic retinopathy in a defined
older population: the Blue Mountains Eye Study. Eye 2007;21(4):465-71.
- New Zealand Guidelines Group (NZGG). New Zealand cardiovascular guidelines handbook. 2nd ed. Wellington: NZGG, 2009.
- Early Treatment Diabetic Reintopathy Study (ETDRS) Research Group. Effects of aspirin treatment on diabetic retinopathy.
ETDRS report number 8. Ophthalmology 1991;98(5 Suppl):757-65.
- The ACCORD Study Group and ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type
2 diabetes. N Engl J Med 2010;363(3):233-44.
- Spurling G, Askew D, Jackson C. Retinopathy screening recommendations. Aust Fam Physician 2009;38(10):780-3.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications
in type 2 diabetes: UKPDS 38. BMJ 1998;317(7160):703-13.
- Keech A, Mitchell P, Summanen P, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy
(FIELD study): a randomised controlled trial. Lancet 2007;370(9600):1687-97.
- Walsh C, Soler N, Fitzgerald M, Malins J. Association of foot lesions with retinopathy in patients with newly diagnosed
diabetes. Lancet 1975;1(7912):878-80.
- Liew G, Klein R, Wong T. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin 2009;49(2):35-52.
- Simo R, Hernandez C. Advances in the medical treatment of diabetic retinopathy. Diabet Care 2009;32(8):1556-62.
- Watkins P. ABC of diabetes: retinopathy. BMJ 2003;326:924-6.
- Goold L, Durkin S, Crompton J. Sudden loss of vision: history and examination. Austr Fam Phy 2009;38(10):764-8.